Molecular Machinery That Delivers Metabolites to Mitochondria
News Release, World Mitochondria Society, Berlin - Germany – May 13, 2022
Metabolites enter the mitochondria.
The process goes far beyond fueling power generation in the cell, according to Hongying Shen, PhD, assistant professor of cellular & molecular physiology at Yale School of Medicine and a member of Yale’s Systems Biology Institute. “Mitochondria also house many other biochemical processes that are critical for cellular and organismal physiology, and that require trafficking in and out of all kinds of metabolites, including nucleotides, amino acids for protein, and lipids,” she says.
In a study published May 5 in Nature Communications, Shen and her lab have identified the molecular machinery through which many of the metabolites reach inside the mitochondria.
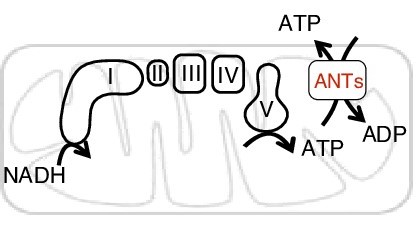
The function of adenine nucleotide carriers in supporting bioenergetics.
They focus on the human SLC25 carrier family, the largest protein family responsible for metabolite translocation across the mitochondrial membrane. Each of the 53 transporters has a distinct assignment. “They are structurally, sequence-wise, very similar to each other,” says Shen, “but they have this amazing specificity. One is dedicated to a certain type of nutrients, the other dedicated to other metabolites or nutrients. So there seems to be a very tight regulation in terms of specificity to recognize metabolites being transported.”
This new knowledge may open the door to potential regulation of what enters the cell, with the goal of preventing or mitigating disease.
A blueprint for treating neurological disorders?
“We are particularly interested in human diseases affecting the brain that include psychiatric disorders and neurodegenerative disorders,” Shen explains. “In fact, there have been de novo mutations in the gene SLC25A39 that have been implicated in autism. And also, A39 has been recently implicated in Parkinson’s disease where oxidative stress was proposed as a pathological mechanism.” In addition, according to Shen, the antioxidant metabolite glutathione, whose delivery route her lab also identified, may be of great interest to scientists studying cancer.
One day in the future, it is conceivable that biomarkers could associate conditions such as neurodegeneration with the metabolic processes that Shen’s lab is studying. That, she says, could lead to new treatments for disease. “Then we can perhaps change our metabolism by diet and by nutrition and all kinds of methods to intervene with that. If we were able to discover these processes and identify the metabolites, can we use dietary intervention to slow the disease onset or disease progression? There's a long way to go [before we might accomplish that], but it's something.”
The new study appears to lay a sound foundation for future work.
Targeting Mitochondria 2022 will cover the most recent studies on role of the mitochondria in health and in disease. It will unravel the latest discoveries and mechanisms in this field with the help of international professional scientists. Meet the speakers.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Balancing lipids and recycling to prevent mitochondrial meltdown
News Release, World Mitochondria Society, Berlin - Germany – April 28, 2022
An international team led by scientists at the University of Helsinki have discovered that the cellular balance of lipid droplets can impact the recycling of damaged mitochondria.
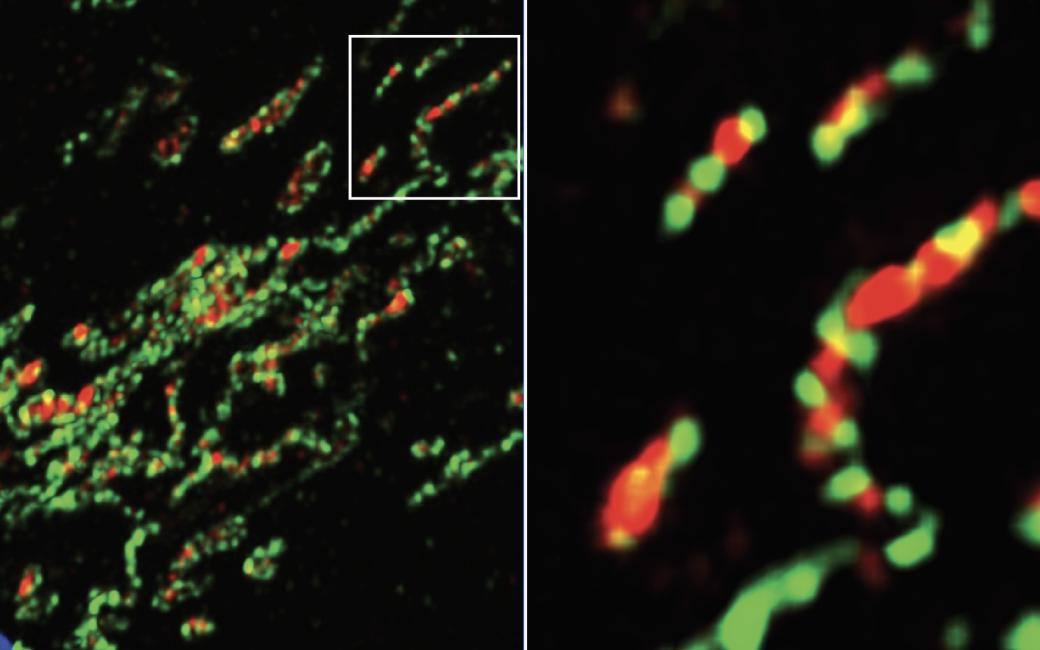
Mitochondria being decorated with eat-me signals. (Image: McWilliams Lab (Fumi Suomi and Homa Ehsan))
Mitochondria release chemical energy and influence metabolic pathways that keep our cells and tissues healthy. Damage to these multifunctional powerhouses promotes cell death and disease. To prevent "mitochondrial meltdown," our cells destroy defective mitochondria using a specialised recycling process termed "mitophagy". Mitophagy is implicated in many diseases and is a major pharmaceutical target for neurodegenerative disorders such as Parkinson's disease.
Unexpected insights into metabolism, mitophagy and movement
The international team of researchers studied a therapeutic molecule used to promote high levels of mitophagy and found that many metabolic pathways involving lipids were rapidly "rewired" before mitochondrial recycling took place.
“Surprisingly, the activity of a protein called DGAT1 is switched on to generate specialised structures known as lipid droplets, typically used to store fat. By impairing DGAT activity, we observed the disappearance of lipid droplets and reduced mitochondrial recycling, and cells were more vulnerable to stress and death,” says Assistant Professor Thomas McWilliams, who led the study.
Remarkably, when the DGAT1 gene was switched off in the brains of reporter flies, both mitophagy and motor function of the animals were severely impacted.
A discovery forged from iron
The study also makes unexpected insights into iron, an essential cofactor for life. The therapeutic molecule used to induce mitophagy is a chelator, a potent drug that depletes cellular iron and researchers found surprisingly rapid effects of its depletion on cellular metabolism. McWilliams says:
"Iron homeostasis represents an ancient function of the mitochondrial network, and iron depletion after many hours promotes mitochondrial recycling. Postdoctoral researcher Maeve Long performed a series of ambitious experiments in my lab, profiling human cells after mere minutes of deferiprone exposure. Our collaborators then mapped very dynamic changes in metabolism in advance of mitophagy. This led us to study lipid crosstalk in further detail, with our Cambridge collaborators highlighting the significance of this synergy in vivo."
Little is known about the factors that regulate physiological mitophagy, and this work opens new avenues for targeting this process. Commenting further, McWilliams adds:
"Defective mitochondrial recycling is problematic for cell types that are very long-lived, such as nerve cells in the brain. Neurodegenerative pathology is often progressive, taking place over many years. When mitophagy is defective, it's reasonable that cells might adapt and utilise additional strategies to stay alive. Much more work is needed, but this is an unexpected and exciting find".
Targeting Mitochondria 2022 will cover the most recent studies on role of the mitochondria in health and in disease. It will unravel the latest discoveries and mechanisms in this field with the help of international professional scientists. Meet the speakers.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Mitochondria Derived “Mitovesicles” in Down Syndrome
News Release, World Mitochondria Society, Berlin - Germany – March 29, 2022
Mitochondrial dysfunction is an established hallmark of aging and neurodegenerative disorders such as Down syndrome (DS) and Alzheimer’s disease (AD).
Using a high-resolution density gradient separation of extracellular vesicles (EVs) isolated from murine and human DS and diploid control brains, D'acunzo et al, were able to identify and characterize a previously unknown population of double-membraned EVs containing multiple mitochondrial proteins distinct from previously described EV subtypes, including microvesicles and exosomes.
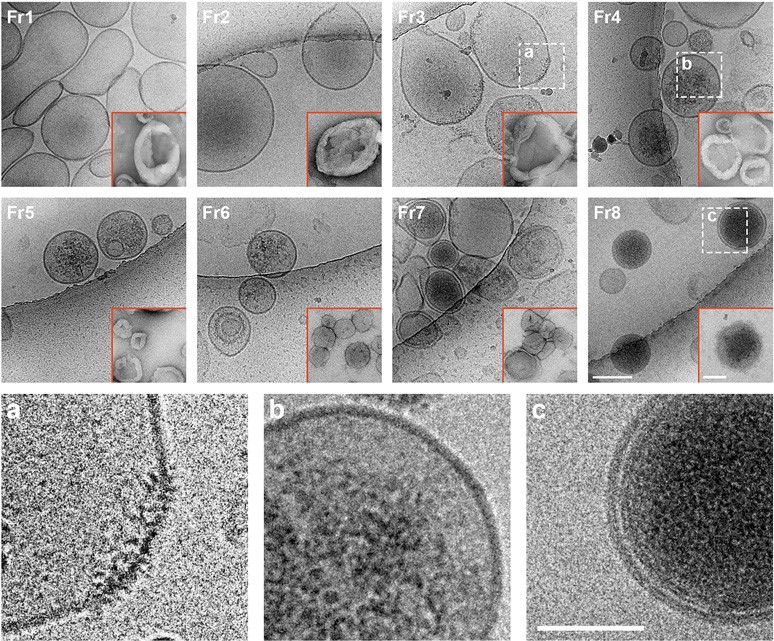
Representative photomicrographs of brain EVs imaged by either cryoEM or TEM (insets with red borders)
They termed these newly identified mitochondria-derived EVs “mitovesicles”.
They demonstrated that brain-derived mitovesicles contain a specific subset of mitochondrial constituents and that their levels and cargo are altered during pathophysiological processes where mitochondrial dysfunction occurs, including in DS.
Thus, the brain cells constitutively secrete mitochondrial vesicles and this pathway is altered under conditions in which mitochondrial dysfunction occurs, providing new insights into heterogeneous EV biology and its relationship with mitochondrial dynamics.
This brilliant discovery opens the door to future development of a method for the selective isolation of mitovesicles paves the way for the characterization in vivo of biological processes connecting EV biology and mitochondria dynamics and for innovative therapeutic and diagnostic strategies.
Targeting Mitochondria 2022 will specialize a whole session entitled “Extracellular Vesicles & Mitochondria: The Target”. Professional speakers like Dr. Ines Khadimallah and Dr. Marc Germain will be sharing with you their experties in this field on October. Join us and remember you still have timw to benefit from the early bird offer.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Mitochondria Transplantation Between Living Cells
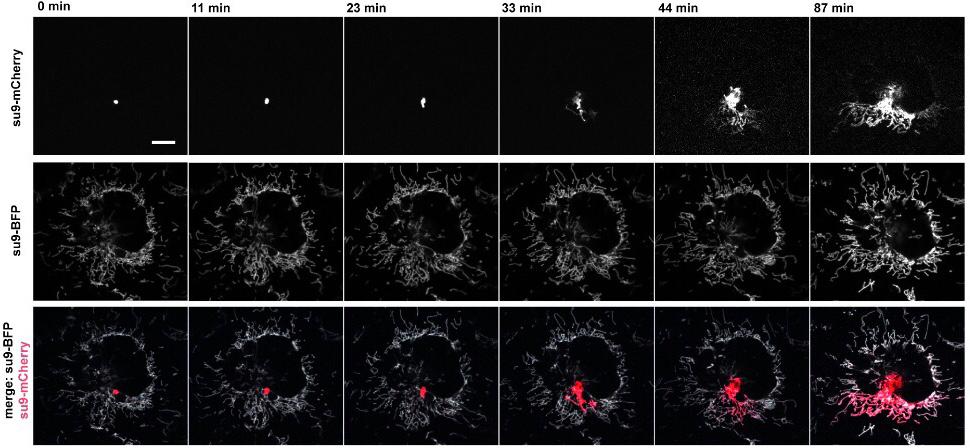
Time-lapse image series of a single transplanted mitochondrion
News Release, World Mitochondria Society, Berlin - Germany – April 6, 2022
Mitochondria and the complex endomembrane system are hallmarks of eukaryotic cells. To date, it has been difficult to manipulate organelle structures within single live cells.
Gäbelein et al, developed a FluidFM-based approach to extract, inject, and transplant organelles from and into living cells with subcellular spatial resolution. The technology combines atomic force microscopy, optical microscopy, and nanofluidics to achieve force and volume control with real-time inspection.
They developed dedicated probes that allow minimally invasive entry into cells and optimized fluid flow to extract specific organelles. When extracting single or a defined number of mitochondria, their morphology transforms into a pearls-on-a-string phenotype due to locally applied fluidic forces. They show that the induced transition is calcium independent and results in isolated, intact mitochondria.
Upon cell-to-cell transplantation, the transferred mitochondria fuse to the host cells mitochondrial network. Transplantation of healthy and drug-impaired mitochondria into primary keratinocytes allowed monitoring of mitochondrial subpopulation rescue. Fusion with the mitochondrial network of recipient cells occurred 20 minutes after transplantation and continued for over 16 hours. After transfer of mitochondria and cell propagation over generations, donor mitochondrial DNA (mtDNA) was replicated in recipient cells without the need for selection pressure.
The approach opens new prospects for the study of organelle physiology and homeostasis, but also for therapy, mechanobiology, and synthetic biology.
In Targeting Mitochondria 2022, a whole session will be focused on Mitochondria Transplantation and Transfer. Professional speakers like Dr. James McCully will be sharing their most recent work in this field of study.
Remember that you too can share your recent reasearch on this topic by submitting your abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Cell Growth & Mitochondrial Function: Where Music Stands
News Release, World Mitochondria Society, Berlin - Germany – March 25, 2022
Mitochondria are considered a portal to receive, process and integrate external energy and information to maintain cellular homeostasis. Previous studies demonstrated that mitochondrial function and antioxidant capacity of in-vitro cultured mammalian cells is modified by different energetic stimuli including electromagnetic energy, acoustic energy, external Qi and subtle energy emitted by written texts in relatively short time frames. Thus, individual cells can act as a rapid and sensitive biological sensor and act as a platform to evaluate and understand the subtle effect of different biophysical stimuli.
Regarding acoustic energy, several different types of music have been reported to produce beneficial effects on human health. Feng et al, compared eastern and western music styles on cell function at the biochemical level to understand the underlying mechanisms involved.
They compared the effects of Chinese five-element music with two types of western music (heavy-metal and classical) on mitochondrial function, oxidative capacity and growth using human embryonic kidney cells (HEK293T cells).
Exposing cells to five-element music produced several beneficial physiological effects including:
- Increases in the production of adenosine triphosphate (ATP) by 17%, glutathione (GSH) by 21% and cell growth rates by 14%
- Significant reduction in the reactive oxygen species (ROS) by 13%.
The group treated with classical music:
- There was only a trend toward increased GSH (8%), although the increased growth rates (14%) did reach significance.
Cells treated with heavy-metal music responded oppositely:
- Significant 16% increase in ROS
- Significant 11% reduction in cell viability
This study revealed dramatically different effects of different styles of music on specific biochemical measures in cultured human cells. It helped explain the underlying biochemical mechanisms of the effects of the different types of music.
Targeting Mitochondria 2022 will introduce to the most recent discoveries on mitochondria. Join us this October and be up to date with all the new research in this field.
© Image - GarryKillian, freepik
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com




























































