Mitochondria, p53, and the Secret to Aging Gracefully.
- Details
- Published on 24 March 2025
As we age, our cells go through stress and damage—especially to their DNA. One of the key proteins that protects us from this damage is p53, often called the “guardian of the genome.” It helps repair broken DNA and prevents damaged cells from turning into cancer.
But this new study, published in Nature Communications, shows that p53 does even more than we thought: it also helps stop inflammation that can build up in aging cells.
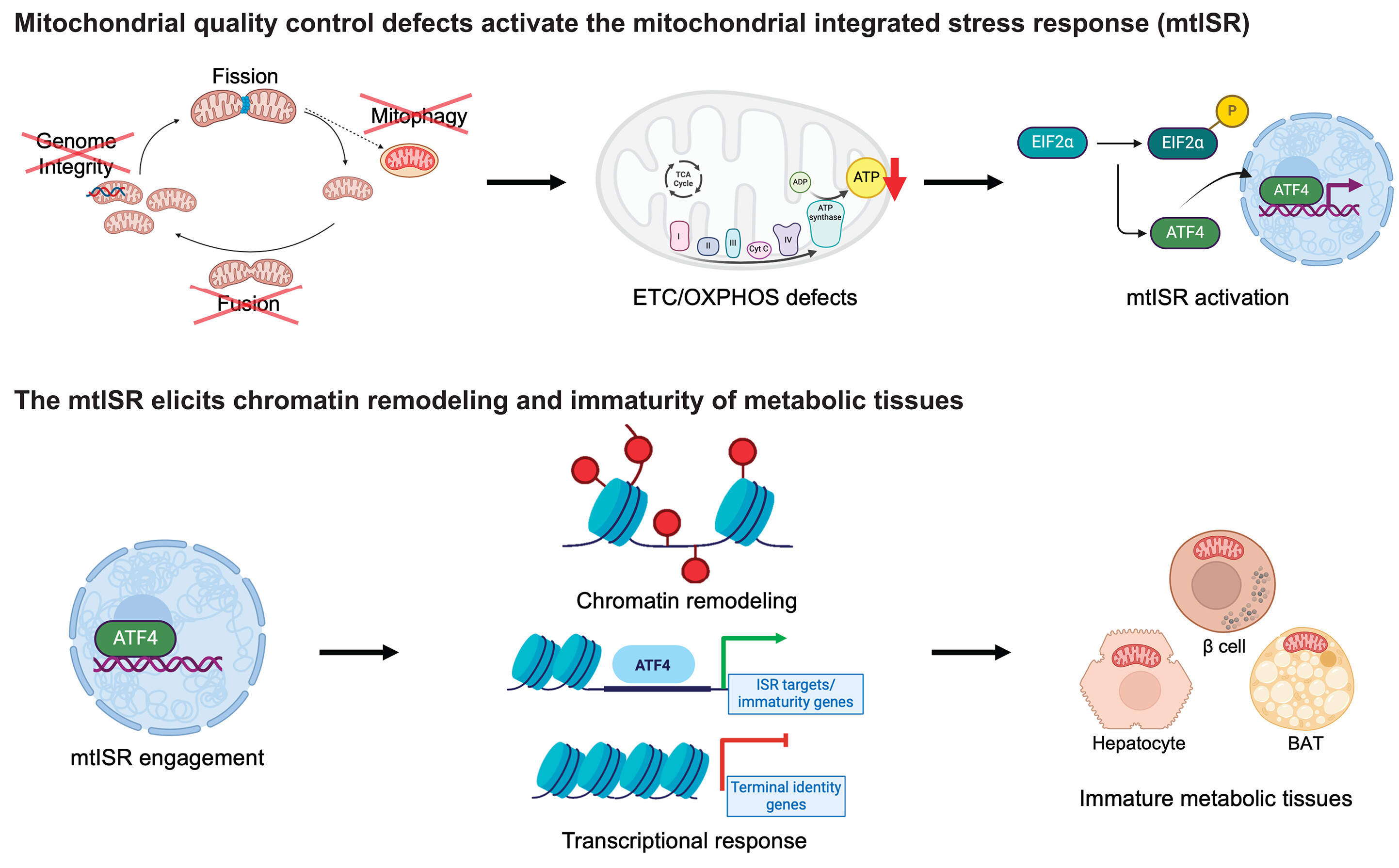
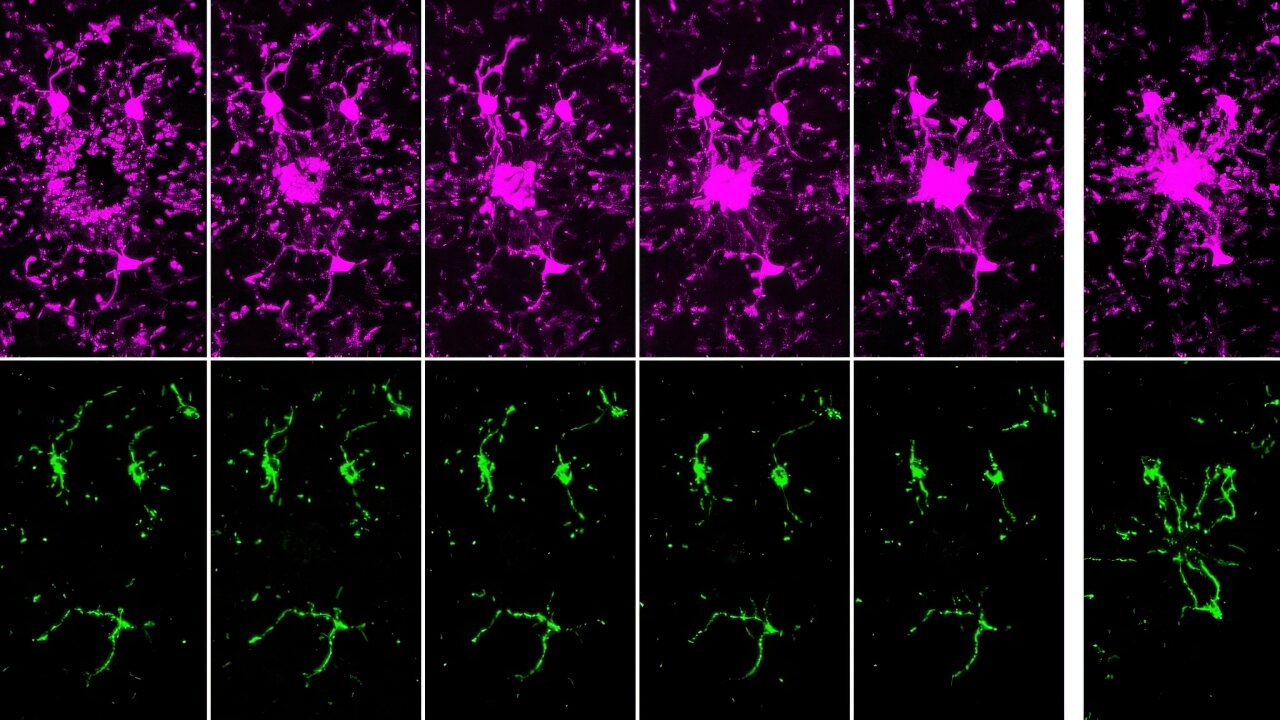
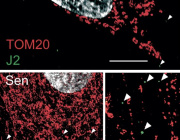
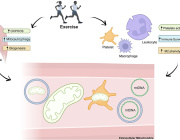
The researchers discovered that mitochondria, which are best known for producing energy, play a key role in controlling how p53 works. When mitochondria function well, they support p53 in repairing DNA and stopping the cell from releasing pieces of damaged DNA—called cytoplasmic chromatin fragments (CCFs)—into the rest of the cell. These fragments can trigger chronic inflammation, a common problem in aging and age-related diseases. By keeping DNA inside the nucleus and helping it stay intact, p53 helps reduce this inflammation and keep the cell stable. When mitochondria aren’t working properly, though, p53 can’t do its job as well—leading to more DNA damage, more CCFs, and more inflammation.
This discovery adds an important piece to the puzzle of aging: it shows how mitochondria and p53 work together to protect cells, maintain DNA integrity, and prevent harmful inflammation. It could open new doors for therapies targeting aging, inflammation, and mitochondrial diseases.
More scientific Details :
Using mitochondrial stress models and p53 loss-of-function systems, the authors show that functional p53:
• Promotes DNA damage repair,
• Limits DNA leakage into the cytoplasm,
• Suppresses cGAS-STING-mediated inflammatory signaling,
• And overall contributes to nuclear genome stability in senescent cells.
Notably, the depletion of p53 led to an increase in nuclear envelope instability and accumulation of CCFs, while its presence correlated with reduced senescence-associated secretory phenotype (SASP) expression. These effects were tightly linked to mitochondrial status, suggesting a mitochondria-to-nucleus signaling axis regulating p53 activity.
This work positions p53 as a key integrator of mitochondrial signals to modulate nuclear architecture and inflammatory outcomes during senescence. It highlights potential therapeutic avenues to modulate mitochondrial-p53 interactions in aging-related pathologies and chronic inflammatory conditions.