Inflammation Discovery Could Slow Aging, Prevent Age-Related Diseases: Strategic Role of Mitochondria

A new discovery from School of Medicine researchers could let us slow the clock on aging and may help us prevent many diseases that plague us in our later years.
School of Medicine researchers have discovered a key driver of chronic inflammation that accelerates aging. That finding could let us slow the clock to live longer, healthier lives, and may allow us to prevent age-related conditions such as deadly heart disease and devastating brain disorders that rob us of our faculties.
So what drives this harmful inflammation? The answer is improper calcium signaling in the mitochondria of certain immune cells. Mitochondria are the power generators in all cells, and they rely heavily on calcium signaling.
The UVA Health researchers, led by Bimal N. Desai, PhD, found that the mitochondria in the immune cells called macrophages lose their ability to take up and use calcium with age. This, the researchers show, leads to chronic inflammation responsible for many of the ailments that afflict our later years.
The researchers believe that increasing calcium uptake by the mitochondrial macrophages could prevent the harmful inflammation and its terrible effects. Because macrophages reside in all organs of our bodies, including the brain, targeting such “tissue-resident macrophages” with appropriate drugs may allow us to slow age-associated neurodegenerative diseases.
“I think we have made a key conceptual breakthrough in understanding the molecular underpinnings of age-associated inflammation,” said Desai, of UVA’s Department of Pharmacology and UVA’s Carter Immunology Center. “This discovery illuminates new therapeutic strategies to interdict the inflammatory cascades that lie at the heart of many cardiometabolic and neurodegenerative diseases.”
The Inflammation of Aging – ‘Inflammaging’
Macrophages are white blood cells that play critical roles in our immune systems and, in turn, our good health. They swallow up dead or dying cells, allowing our bodies to remove cellular debris, and patrol for pathogens and other foreign invaders. In this latter role, they act as important sentries for our immune systems, calling for help from other immune cells as needed.
Scientists have known that macrophages become less effective with age, but it has been unclear why. Desai’s new discovery suggests answers.
Desai and his team say their research has identified a “keystone” mechanism responsible for age-related changes in the macrophages. These changes, the scientists believe, make the macrophages prone to chronic, low-grade inflammation at the best of times. And when the immune cells are confronted by an invader or tissue damage, they can become hyperactive. This drives what is known as “inflammaging” – chronic inflammation that drives aging.
Further, the UVA Health scientists suspect that the mechanism they have discovered will hold true not just for macrophages but for many other related immune cells generated in the bone marrow. That means we may be able to stimulate the proper functioning of those cells as well, potentially giving our immune systems a big boost in old age, when we become more susceptible to disease.
Next Steps
Fixing “inflammaging” won’t be as simple as taking a calcium supplement. The problem is not a shortage of calcium so much as the macrophages’ inability to use it properly. But Desai’s new discovery has pinpointed the precise molecular machinery involved in this process, so we should be able to discover ways to stimulate this machinery in aging cells.
“This highly interdisciplinary research effort, at the interface of computational biology, immunology, cell biology and biophysics, wouldn’t have been possible without the determination of Phil Seegren, the graduate student who spearheaded this ambitious project,” Desai said. “Now, moving forward, we need an equally ambitious effort to figure out the wiring that controls this mitochondrial process in different types of macrophages and then manipulate that wiring in creative ways for biomedical impact.”
Aging Findings Published
The researchers have published their findings in the scientific journal Nature Aging. The article is open access, meaning it is free to read.
The research team consisted of Philip V. Seegren, Logan R. Harper, Taylor K. Downs, Xiao-Yu Zhao, Shivapriya B. Viswanathan, Marta E. Stremska, Rachel J. Olson, Joel Kennedy, Sarah E. Ewald, Pankaj Kumar and Desai. The scientists reported that they have no financial interests in the work.
The research was supported by the National Institutes of Health, grants AI155808, GM108989, GM138381, P30 CA044579 and T32 GM007055-46, and by the Owens Family Foundation.
Source: UVA Health
Targeting Mitochondria 2023 will introduce you to the latest research on mitochondria and longevity. Submit a related abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Artificial Mitochondria: The Dream is Here
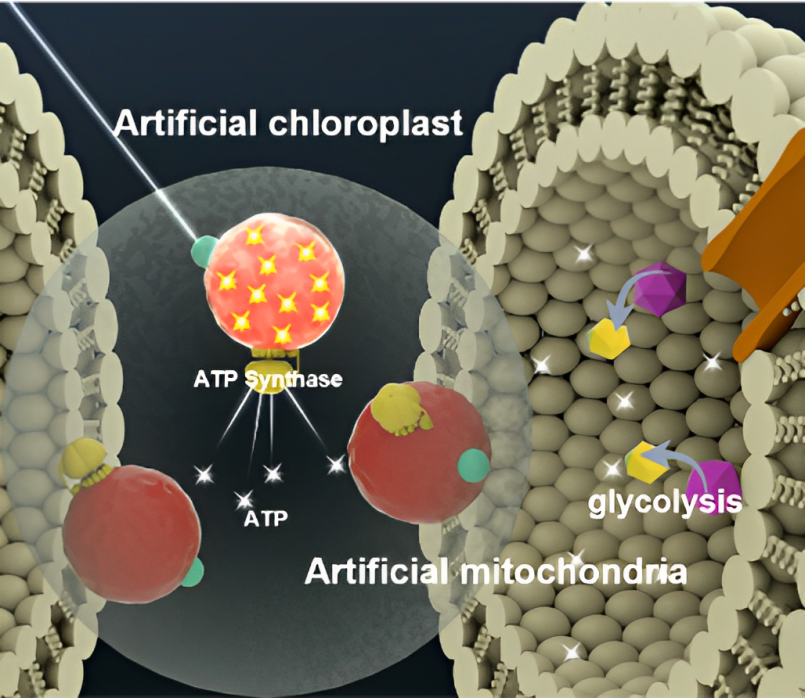
Concept of artificial chloroplasts and mitochondria within a liposome for self-sustaining energy generation through photosynthesis and cellular respiration.
Energy production in nature is the responsibility of chloroplasts and mitochondria and is crucial for fabricating sustainable, synthetic cells in the lab. Mitochondria are not only "the powerhouses of the cell," as the middle school biology adage goes, but also one of the most complex intracellular components to replicate artificially.
In Biophysics Reviews, by AIP Publishing, researchers from Sogang University in South Korea and the Harbin Institute of Technology in China identified the most promising advancements and greatest challenges of artificial mitochondria and chloroplasts.
"If scientists can create artificial mitochondria and chloroplasts, we could potentially develop synthetic cells that can generate energy and synthesize molecules autonomously. This would pave the way for the creation of entirely new organisms or biomaterials," author Kwanwoo Shin said.
In plants, chloroplasts use sunlight to convert water and carbon dioxide into glucose. Mitochondria, found in plants and animals alike, produce energy by breaking down glucose.
Once a cell produces energy, it often uses a molecule called adenosine triphosphate (ATP) to store and transfer that energy. When the cell breaks down the ATP, it releases energy that powers the cell's functions.
"In other words, ATP acts as the main energy currency of the cell, and it is vital for the cell to perform most of the cellular functions," said Shin.
The team describes the components required to construct synthetic mitochondria and chloroplasts and identifies proteins as the most important aspects for molecular rotary machinery, proton transport, and ATP production.
Previous studies have replicated components that make up the energy-producing organelles. Some of the most promising work investigates the intermediate operations involved in the complex energy-generating process. By connecting the sequence of proteins and enzymes, researchers have improved energy efficiency.
One of the most significant challenges remaining in trying to reconstruct the energy production organelles is enabling self-adaptation in changing environments to maintain a stable supply of ATP. Future studies must investigate how to improve upon this limiting feature before synthetic cells are self-sustainable.
The authors believe it is important to create artificial cells with biologically realistic energy-generation methods that mimic natural processes. Replicating the entire cell could lead to future biomaterials and lend insight into the past.
"This could be an important milestone in understanding the origin of life and the origin of cells," Shin said.
Source: Biophysics Reviews (AIP Publishing)Targeting Mitochondria 2023 will introduce you to the latest mitochondria discoveries and innovations. Submit a related abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Distinct Longevity Mechanisms Across and Within Species & Their Association With Aging
News Release, World Mitochondria Society, Berlin - Germany – June 26, 2023
Lifespan varies within and across species, but the general principles of its control remain unclear.
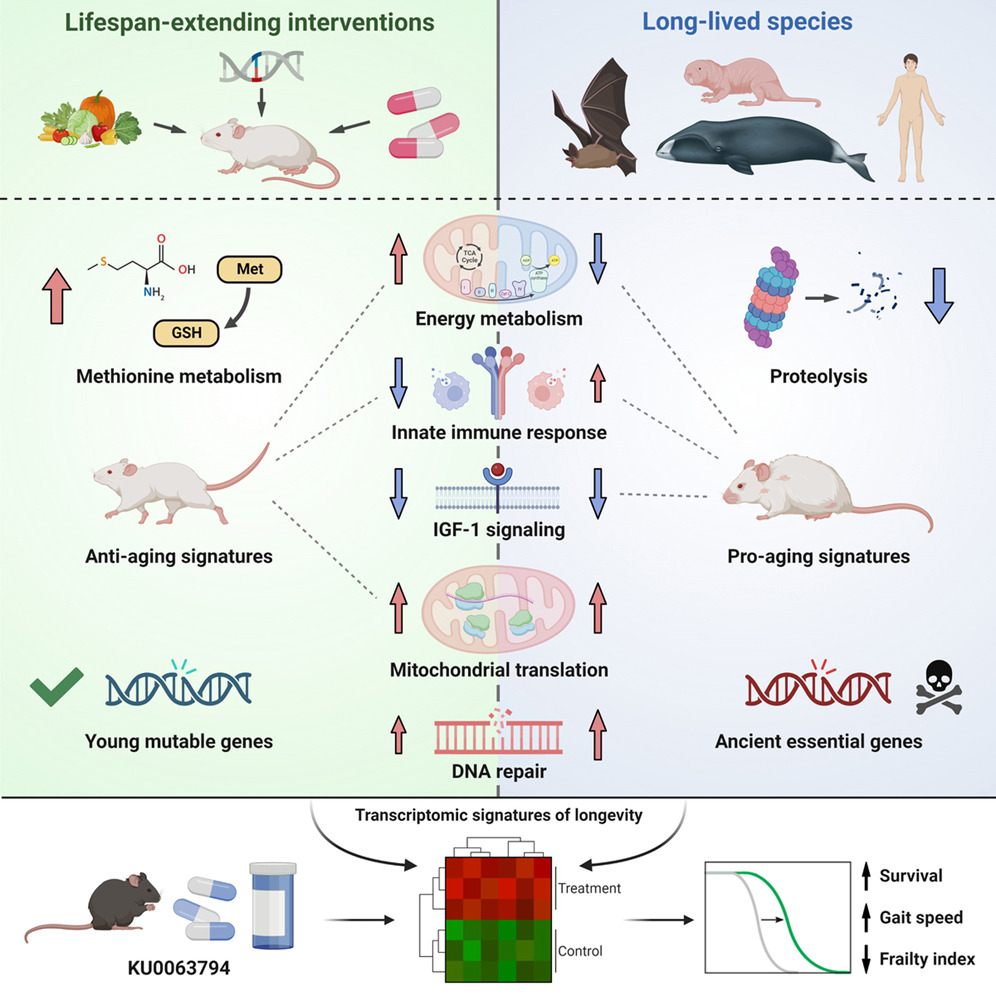
In their new study, published in Cell, Vadim N. Gladyshev from Harvard Medical school and his team, conducted multi-tissue RNA-seq analyses across 41 mammalian species, identifying longevity signatures and examining their relationship with transcriptomic biomarkers of aging and established lifespan-extending interventions.
An integrative analysis uncovered shared longevity mechanisms within and across species, including downregulated Igf1 and upregulated mitochondrial translation genes, and unique features, such as distinct regulation of the innate immune response and cellular respiration.
Signatures of long-lived species were positively correlated with age-related changes and enriched for evolutionarily ancient essential genes, involved in proteolysis and PI3K-Akt signaling. Conversely, lifespan-extending interventions counteracted aging patterns and affected younger, mutable genes enriched for energy metabolism. The identified biomarkers revealed longevity interventions, including KU0063794, which extended mouse lifespan and healthspan.
In summary their research highlighted that:
- Distinct molecular mechanisms control lifespan within and across species
- Aging effects are reversed by longevity interventions but not by species longevity
- Regulation of Igf1 and mitochondrial translation are shared signatures of longevity
- Longevity signatures enable the discovery of geroprotectors, such as KU0063794
Overall, this study uncovers universal and distinct strategies of lifespan regulation within and across species and provides tools for discovering longevity interventions.
Image Credits: Tyshkovskiy, Alexander et al. Cell, Volume 186, Issue 13, 2929 - 2949.e20
Targeting Mitochondria 2023 this October will dedicate a full session to "Mitochondria & Longevity: Towards Expanding Life Span". Submit a related abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Heart tissue heads to space to aid research on aging and impact of long spaceflights

Johns Hopkins Medicine researchers are collaborating with NASA to send human heart “tissue-on-a-chip” specimens into space as early as March. The project is designed to monitor the tissue for changes in heart muscle cells’ mitochondria (their power supply) and ability to contract in low-gravity conditions.
The tissue samples will be launched into space aboard SpaceX CRS-27, a resupply mission to the International Space Station, slated for liftoff no earlier than Tuesday, March 14, at NASA’s Kennedy Space Center in Florida.
Astronauts on board during the mission will also introduce three FDA-approved medicines to the samples in efforts to prevent heart cell changes known or suspected to occur in those undertaking long-duration spaceflights.
“It’s possible that what we learn from these experiments in space could also inform how we treat age-related cardiac problems,” says Deok-Ho Kim, professor of biomedical engineering at the Johns Hopkins University School of Medicine, because many heart cellular changes already detected in space explorers mimic changes linked to heart muscle aging in general.
To develop the microengineered human heart tissue-on-a-chip, researchers begin with human induced pluripotent stem cells grown in the laboratory. Such cells are able to develop into nearly any type of cell, and are coaxed biologically to develop into beating human cardiomyocytes, the muscle cells that make hearts contract.
Groups of cardiomyocytes form tissue that can be strung between two posts, one flexible and one stiff. The flexible post has an embedded magnet and, when placed over sensors, allows for collection of information on tissue contraction. The chamber enclosing the tissue is sealed so that liquid media feeding the tissue doesn’t float away in space. These tissue chambers are then loaded into so-called plate habitats with the magnetic sensors located beneath the tissue. The experimental payload consists of two of these plate habitats, which measure about 7 inches long, 5 inches tall and 4 inches wide.
Kim, his previous postdoctoral researcher Jonathan Tsui, and his doctoral student Devin Mair previously sent heart tissue into space in March 2020. Those experiments, presented at the Tissue Engineering and Regenerative Medicine International Society-Americas 2022 Annual Meeting, showed that microgravity in space changed the cells’ mitochondria and the tissues’ ability to contract.
In the new experiments with their microengineered human heart tissues-on-a-chip, the scientists will focus on the proteins activated during tissue inflammation and mitochondrial dysfunction.
The astronauts aboard the space station will also test whether any of three medicines can stave off the problems anticipated in space-bound heart cells.
Source: John Hopkins School of Medicine
Targeting Mitochondria 2023 will dedicate a whole session to "Mitochondria, Microgravity & Space Travel – A glance into the future". Submit a related abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Severe SARS-CoV-2 Infection as a Marker of Undiagnosed Cancer
News Release, World Mitochondria Society, Berlin - Germany – June 7, 2023
This population-based study by Dugerdil et al, published in scientific reports, investigated if a severe SARS-CoV-2 infection represents a marker of an undiagnosed cancer.
The SNDS database was used, identified from 02/15/2020 to 08/31/2021, 41,302 individuals hospitalized in intensive care unit due to SARS-CoV-2 (ICU-gr) and 713,670 control individuals not hospitalized for SARS-CoV-2 (C-gr). Individuals were matched according to year of birth, sex and French department.The cancer incidence was compared in the two groups during the follow-up period, using Cox proportional hazards models adjusted on matching variables, socioeconomic characteristics and comorbidities.
In the ICU-gr, 2.2% was diagnosed with a cancer in the following months, compared to 1.5% in the C-gr. The ICU-gr had a 1.31 higher risk of being diagnosed with a cancer following hospital discharge compared to the C-gr. A global similar trend was found when competing risk of death was taken into account. A significant higher risk was found concerning renal, hematological, colon, and lung cancers.
The obtained results suggest that a severe SARS-CoV-2 infection may represent a marker of an undiagnosed cancer.
Image credits: by kjpargeter on Freepik
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com




























































