Study Discovers Single-Celled Gut Protists Flourishing Without Mitochondria
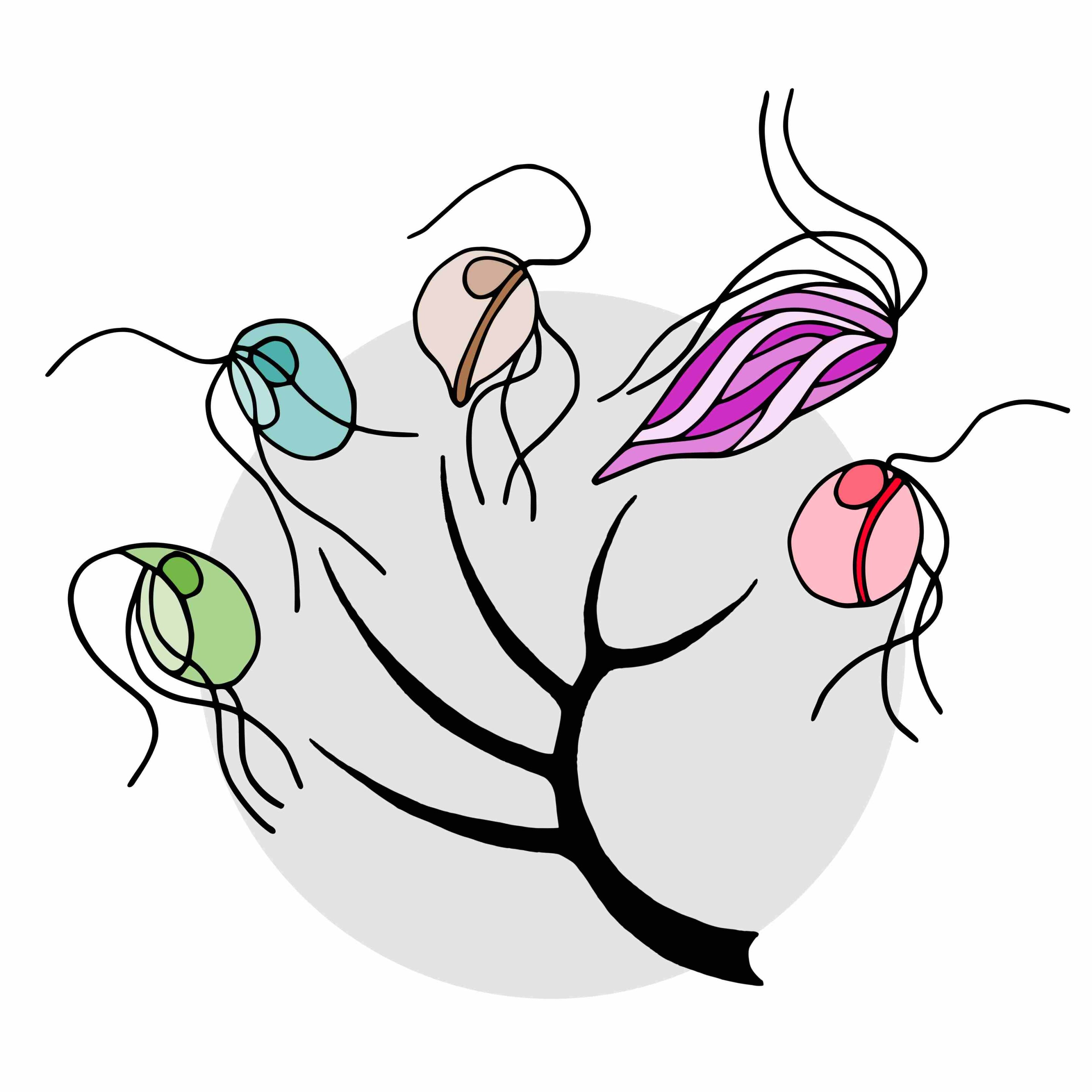
Schematic evolutionary tree of the five microbial species included in the study. From left to right: Trimastix marina, Paratrimastix pyriformis, Blattamonas nauphoetae, Streblomastix strix, and Monocercomonoides exilis.
In a groundbreaking discovery by Lukáš Novák and Vladimír Hampl of Charles University, published in the journal PLOS Genetics, Oxymonadida flagellates, a group of single-celled protists that live inside the guts of termites and other animals including Blattamonas nauphoetae and Monocercomonoides exilis, challenge the conventional belief that mitochondria are indispensable.
High-quality genome analysis revealed the complete loss of mitochondria in three oxymonad species, pushing this unique event back 100 million years ago.
-
Mitochondrial Loss Across Species: Genomic studies confirm the absence of mitochondria in Blattamonas nauphoetae, Streblomastix strix, and Monocercomonoides exilis, suggesting this trait is common to the entire Oxymonadida group.
-
Evolutionary Insights: The timeline of mitochondrial loss places this phenomenon at around 100 million years ago, coinciding with the diversification of the oxymonad lineage.
-
Metabolic Adaptations: Comparative analysis highlights the metabolic changes accompanying the transition to amitochondriality, shedding light on the unique evolutionary journey of oxymonads.
In summary, Oxymonad flagellates challenge our understanding of cellular evolution by completely shedding mitochondria, marking a significant paradigm shift in eukaryotic biology. The study's insights into the ancient origins and metabolic adaptations of these organisms provide a fascinating glimpse into the mysteries of their evolutionary past.
Photo Credits: Lukas Novak, CC-BY 4.0, https://creativecommons.org/licenses/by/4.0/)
World Congress on Targeting Mitochondria
World Mitochondria Society
wms-site.com
Blood Tests Uncover Biomarkers Linked to Suicidal Thoughts
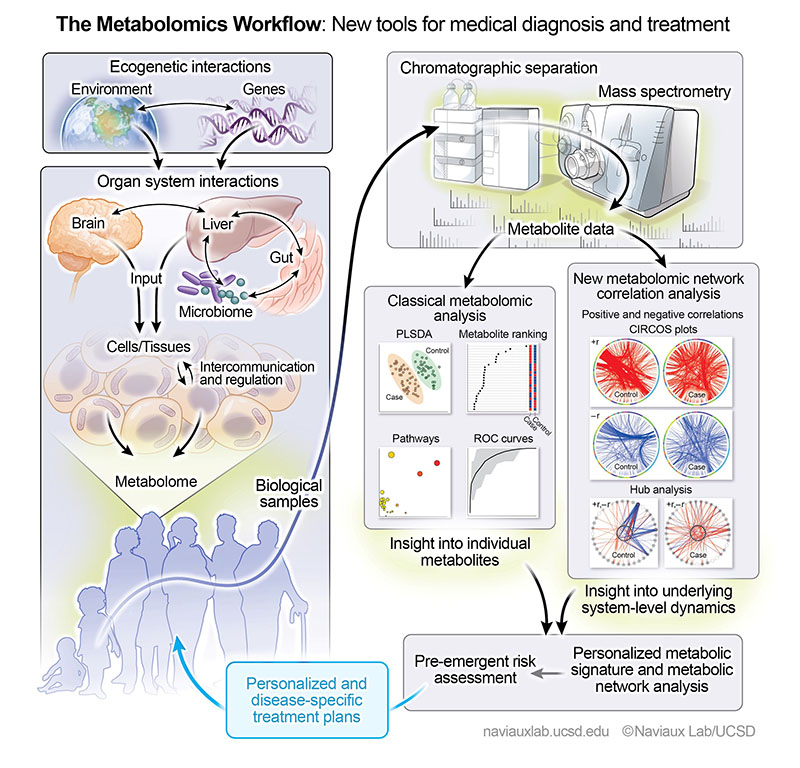
This graphic describes the metabolomics workflow the researchers used to analyze the blood of people with depression and suicidal ideation.
Recent research from UC San Diego has identified biomarkers in blood tests that could indicate a higher risk of suicidal thoughts in individuals with depression. This pioneering study, published in "Translational Psychiatry," highlights the significant link between cellular metabolism and mental health. The findings suggest the potential for more tailored treatments and new drug targets, especially focusing on mitochondrial dysfunction. Such advancements could revolutionize the approach to mental health care, offering more personalized and effective solutions.
"The interplay between mitochondria and microbiota in medicine, particularly their impact on mental health, is a fascinating area of focus. This will be a key discussion topic at the Berlin Mitochondria Meeting this October, as highlighted by Dr. Marvin Edeas, president of the World Mitochondria Society's scientific board. This topic is particularly relevant in light of the recent UC San Diego research and findings.
For more details on this groundbreaking research, read the full article.
Photo Credits: UC San Diego Health Sciences
World Congress on Targeting Mitochondria
World Mitochondria Society
wms-site.com
Decoding Chronic Fatigue: The Crucial Role of WASF3 in Mitochondrial Dysfunction in ME/CFS
News Release, World Mitochondria Society, Berlin - Germany – August 15, 2023
A significant study was reported in the Proceedings of the National Academy of Sciences yesterday, shedding new light on the molecular mechanisms underlying chronic fatigue and strategic role of mitochondria. Here are the main takeaways from the study:
Main Finding
In myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), a protein called WASF3, induced by endoplasmic reticulum (ER) stress, detrimentally affects mitochondrial functions, resulting in exercise intolerance.
Patient Case
In a detailed examination of a 38-year-old woman suffering from chronic fatigue, an overexpression of WASF3 was identified. This overexpression is linked to a disruption in the formation of mitochondrial respiratory supercomplexes.
Mouse Model Insights
- Mice with heightened WASF3 levels exhibited reduced exercise capabilities.
- A clear association was observed between elevated WASF3 levels and compromised mitochondrial functionality in skeletal muscles.
Mitigation Strategy
The study suggests a potential intervention. By diminishing ER stress, WASF3 levels were reduced, leading to a noticeable improvement in mitochondrial function.
Extended Research
Further corroborating the primary findings, muscle biopsies from a wider group of ME/CFS patients also presented elevated WASF3 levels, coupled with signs of aberrant ER stress activation.
Broader Implications
Beyond the primary focus on ME/CFS, the findings from this research provide valuable insights that could inform understanding of other fatigue-related disorders. This includes conditions like rheumatic diseases and the emerging concerns surrounding long COVID.
This study marks a pivotal step forward in understanding the intricacies of chronic fatigue at the molecular level, offering hope for improved diagnostic and therapeutic approaches in the future.
Don't miss Trageting Mitochondria 2023 this November in Berlin to stay updated with the latest findings on mitochondria and fatigue. Learn more about the topics that will be covered during Targeting Mitochondria 2023.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Mitochondrial Inhibitors Extend Lifespan in C. elegans: Insights from a Longevity Study
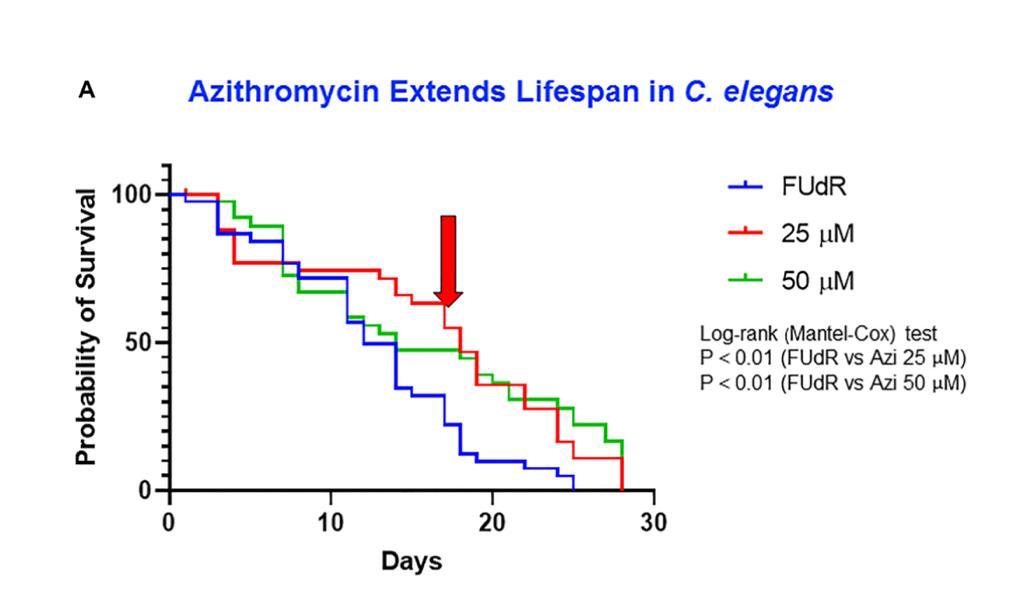
Doxycycline extends lifespan of C. elegans
News Release, World Mitochondria Society, Berlin - Germany – November 27, 2023
Aging, characterized as a progressive degeneration of cell and tissue functions, might be significantly influenced by mitochondrial processes.
Bonuccelli et al., from the University of Salford, United Kingdom used Caenorhabditis elegans (C. elegans) to explore the effects of mitochondrial inhibitors on aging.
Key Findings:
- Treatment with doxycycline and azithromycin (mitochondrial ribosome inhibitors) notably increased C. elegans' median lifespan.
- Improvements were observed in pharyngeal muscle function and a reduction in lipofuscin accumulation.
- ATP consumption was lowered in treated worms, indicating reduced energy expenditure.
- DPI, an inhibitor of mitochondrial complex I and II, also prolonged the median lifespan.
- Conversely, vitamin C treatment did not extend lifespan and resulted in higher ATP levels.
Conclusion: The authors suggest that mitochondrial inhibitors can effectively extend lifespan and counteract aging-related declines in C. elegans, highlighting their potential in aging research.
Photo Credits: Bonuccelli et al. Aging (Albany NY). 2023
World Congress on Targeting Mitochondria
World Mitochondria Society
wms-site.com
Winners of the 2023 Lurie Prize in Biomedical Sciences: Unveiling Distinct Discoveries in Mitochondrial Science

With great admiration, the WMS would like to extend its most sincere and heartfelt congratulations to Navdeep S. Chandel, Ph.D., and Vamsi Mootha, MD. Their relentless dedication and pioneering research in the field of mitochondrial science have not only advanced our understanding of this vital area but have also garnered them the honor of being named the recipients of the highly prestigious 2023 Lurie Prize in Biomedical Sciences.
Each of Dr. Chandel and Dr. Mootha has made important and distinct discoveries in the field of mitochondrial science by exploring the characteristics and functions of mitochondria in human physiology and disease.
Dr. Navdeep Chandel is the David W. Cugell Professor of Medicine, Biochemistry, and Molecular Genetics at Northwestern University Feinberg School of Medicine. The Chandel research team has shown that mitochondria do much more than supply energy to cells. His research team has revealed how mitochondria function as signaling organelles that control the body’s normal functions and impact diseases, including cancer and inflammation.
Dr. Vamsi Mootha is an investigator of the Howard Hughes Medical Institute, investigator in the Department of Molecular Biology at Massachusetts General Hospital, a member of the Broad Institute of MIT and Harvard, and a professor of Systems Biology and Medicine at Harvard Medical School. His laboratory team combines genomics and computation with classic biochemistry and physiology to gain a holistic view of the genes and proteins relevant to mitochondrial function. Although mitochondria contain their own DNA that encodes just 13 proteins, the Mootha research team has identified the other 99% of mitochondrial proteins encoded by nuclear DNA and compiled their findings in a widely used reference tool used to discover new protein functions and disease genes.
The impact of their work will undoubtedly resonate throughout the scientific community and beyond for years to come.
Source: FNIH Press Release
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
More Articles...
- New Outstanding Study Linking Post-Covid to Mitochondrial Damage
- Maternal and Fetal Mitochondrial Gene Dysregulation in Hypertensive Disorders of Pregnancy
- Freiburg research team casts light on signal-dependent formation of mitochondria
- Hydrogen Sulfide: A Promising Healthy ageing Therapeutic When Specifically Targeted Within Cells




























































