Unlocking the Role of Mitochondria in Processing Dietary Fats
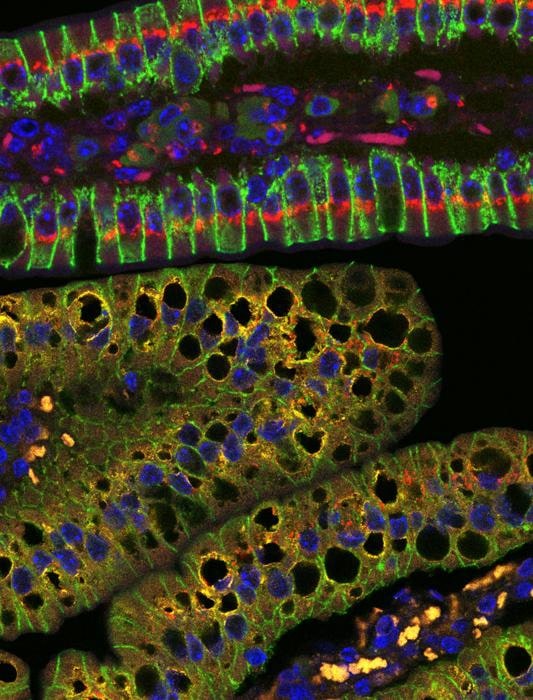
PICTURES SHOW SMALL INTESTINAL VILLI FROM WILD TYPE (TOP) AND ENTEROCYTE-SPECIFIC DARS2 KNOCKOUT MICE (BOTTOM) STAINED FOR PLIN2 (YELLOW), TGN38 (RED), E-CADHERIN (GREEN) AND DNA (DAPI, BLUE).
The maintenance of a balanced lipid homeostasis is critical for our health. While consumption of excessive amounts of fatty foods contributes to metabolic diseases such as obesity and atherosclerosis, fat is an indispensable component of our diet. Digested lipids supply the body with essential building blocks and facilitate the absorption of important vitamins. In a new study published in the journal Nature, a team of researchers led by Professor Manolis Pasparakis and their collaborators Professor Aleksandra Trifunovic and Professor Christian Frezza at the Excellence Cluster CECAD of the University of Cologne, and Professor Jörg Heeren at the University of Hamburg, report on a new mechanism that regulates the processing and transport of dietary lipids by the intestine.
The researchers studied the function of mitochondria – organelles acting as powerhouses of the cell – in enterocytes, cells that line the intestine and specialize in the absorption and transport of nutrients from digested food. They found that disruption of mitochondrial function in the intestines of mice caused abnormal accumulation of dietary fat in enterocytes and impaired delivery of lipids to the peripheral organs.
A key finding of the study was that, when mitochondria did not function properly, enterocytes showed impaired packaging and transport of lipids in the form of chylomicrons. Chylomicrons are crucial carriers of dietary fats, and their proper formation and transport are essential for the absorption of nutrients.
“This discovery marks a significant leap forward in understanding the crucial role of mitochondria in dietary lipid transport and metabolism,” said Dr Chrysanthi Moschandrea, the lead author of the study. The implications of this discovery go beyond the realm of basic research. “These findings provide new perspectives for the better understanding of the gastrointestinal symptoms in patients suffering from mitochondrial disease, and may also lead to new therapeutic approaches,” added Professor Aleksandra Trifunovic.
The maintenance of a balanced lipid homeostasis is critical for our health. While consumption of excessive amounts of fatty foods contributes to metabolic diseases such as obesity and atherosclerosis, fat is an indispensable component of our diet. Digested lipids supply the body with essential building blocks and facilitate the absorption of important vitamins. In a new study published in the journal Nature, a team of researchers led by Professor Manolis Pasparakis and their collaborators Professor Aleksandra Trifunovic and Professor Christian Frezza at the Excellence Cluster CECAD of the University of Cologne, and Professor Jörg Heeren at the University of Hamburg, report on a new mechanism that regulates the processing and transport of dietary lipids by the intestine.
The researchers studied the function of mitochondria – organelles acting as powerhouses of the cell – in enterocytes, cells that line the intestine and specialize in the absorption and transport of nutrients from digested food. They found that disruption of mitochondrial function in the intestines of mice caused abnormal accumulation of dietary fat in enterocytes and impaired delivery of lipids to the peripheral organs.
A key finding of the study was that, when mitochondria did not function properly, enterocytes showed impaired packaging and transport of lipids in the form of chylomicrons. Chylomicrons are crucial carriers of dietary fats, and their proper formation and transport are essential for the absorption of nutrients.
“This discovery marks a significant leap forward in understanding the crucial role of mitochondria in dietary lipid transport and metabolism,” said Dr Chrysanthi Moschandrea, the lead author of the study. The implications of this discovery go beyond the realm of basic research. “These findings provide new perspectives for the better understanding of the gastrointestinal symptoms in patients suffering from mitochondrial disease, and may also lead to new therapeutic approaches,” added Professor Aleksandra Trifunovic.
Photo Credits: Chrysanthi Moschandrea
HKDC1 Ensures Mitochondrial and Lysosomal Balance, Safeguarding Against Cellular Senescence
Osaka, Japan – Just as healthy organs are vital to our well-being, healthy organelles are vital to the proper functioning of the cell. These subcellular structures carry out specific jobs within the cell, for example, mitochondria power the cell and lysosomes keep the cell tidy.
Although damage to these two organelles has been linked to aging, cellular senescence, and many diseases, the regulation and maintenance of these organelles has remained poorly understood. Now, researchers at Osaka University have identified a protein, HKDC1, that plays a key role in maintaining these two organelles, thereby acting to prevent cellular aging.
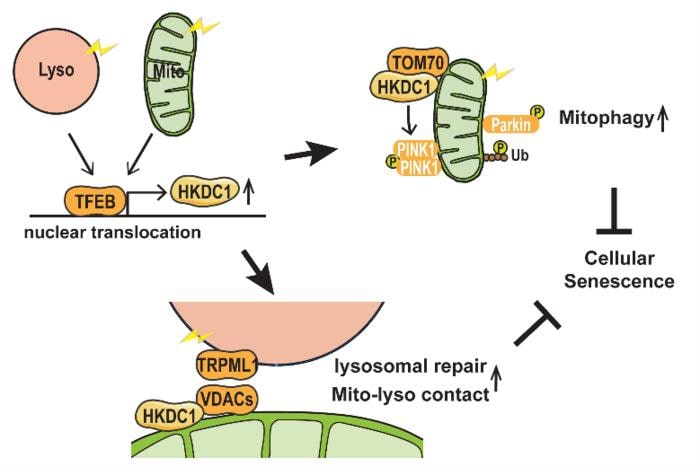
There was evidence that a protein called TFEB is involved in maintaining the function of both organelles, but no targets of this protein were known. By comparing all the genes of the cell that are active under particular conditions, and by using a method called chromatin immunoprecipitation, which can identify the DNA targets of proteins, the team were the first to show that the gene encoding HKDC1 is a direct target of TFEB, and that HKDC1 becomes upregulated under conditions of mitochondrial or lysosomal stress.
One way that mitochondria are protected from damage is through the process of “mitophagy”, the controlled removal of damaged mitochondria. There are various mitophagy pathways, and the most well-characterized of these depends on proteins called PINK1 and Parkin.
“We observed that HKDC1 co-localizes with a protein called TOM20, which is located in the outer membrane of the mitochondria,” explains lead author Mengying Cui, “and through our experiments, we found that HKDC1, and its interaction with TOM20, are critical for PINK1/Parkin-dependent mitophagy.”
So, put simply, HKDC1 is brought in by TFEB to help take out the mitochondrial trash. But what about lysosomes? Well, TFEB and KHDC1 are key players here, too. Reducing HKDC1 in the cell was shown to interfere with lysosomal repair, indicating that HKDC1 and TFEB help lysosomes to recover from damage.
“HKDC1 is localized to the mitochondria, right? Well, this turns out to also be critical for the process of lysosomal repair,” explains senior author Shuhei Nakamura. “You see, lysosomes and mitochondria contact each other via proteins called VDACs. Specifically, HKDC1 is responsible for interacting with the VDACs; this protein is essential for mitochondria–lysosome contact, and thus, lysosomal repair.”
These two diverse functions of HKDC1, with key roles in both the lysosome and the mitochondria, help to prevent cellular senescence by simultaneously maintaining the stability of these two organelles. As dysfunction of these organelles is linked to aging and age-related diseases, this discovery opens new avenues for therapeutic approaches to these diseases.
Photo Credits: 2024 Cui et al., HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence, PNAS.
Zen and the art of mitochondrial maintenance: The machinery of death makes a healthier life
While we all aspire for a long lifespan, what is most coveted is a long period of vigor and health, or “healthspan,” that precedes the inevitable decline of advancing age. Researchers at UC Santa Barbara have discovered that instruments of death that cells use to commit suicide when things go wrong contribute to making a longer and healthier life by revitalizing the specialized cellular compartments called mitochondria.
Mitochondria generate the energy for all of our activities, from movement to thought. These power plants inside our cells descended from what were once free-living bacteria.
“We are a sort of hybrid creature that arose from two independent evolutionary lineages: mitochondria, which were once bacteria, and the rest of the cell surrounding them,” notes Joel Rothman, a professor of molecular biology whose lab conducted the research.
This dual evolutionary origin means that our DNA resides in two separate compartments in each of our cells: the nucleus, where most of our genome is located, and the mitochondria with their own DNA, as a remnant of their bacterial provenance.
“As we age, damage to the DNA in these cellular power houses accumulates, contributing to age-related decline,” notes Rothman. “Our discovery reveals a way that defective mitochondria are removed, resulting in rejuvenation of cells.”
The research, recently published in the journal eLife, shows that the biological machinery that functions as a “kill switch” for cells that are potentially harmful, for example those becoming cancerous, also eliminate the defective DNAs of mitochondria.
“There is a Yin and Yang to mitochondria,” said Pradeep Joshi, a senior scientist and co-author on the publication. “They produce the energy that sustains life. But with every breath, mitochondria also produce reactive oxygen species, harmful molecules that damage DNA and other parts of our cells.”
Thus, the longer we live, the more damage that occurs. This damage diminishes energy production by mitochondria, with negative consequences for our healthspan. As the heart, muscles, and brain demand the most from this energy supply, aging is inevitably associated with heart failure, loss of muscle function, and dementia.
According to Joshi, “aging can be considered a sort of mitochondrial disease. If we could clear out mitochondrial damage, we would improve healthspan and longevity.”
The research team discovered a system for clearing out damaged mitochondria by using a diminutive worm called C. elegans, renowned for many advances in biomedicine, including those recognized by six Nobel prizes.
The researchers found that enzymes responsible for killing cells are also required to remove damaged mitochondrial DNA. In the absence of these enzymes, defective mitochondria pile up.
Rothman and coworkers were surprised to find that, although some of the same proteins are involved, the overall process of removing the damaged mitochondria is different from that normally used to remove excess cells. “The machinery for cell death appears to be repurposed to clear out bad mitochondria,” observed Joshi. “By doing so, they restore the health to these vital power houses.”
As a human, you inherited your mitochondrial DNA exclusively from your mother and the same is true for the animals used in the study. The scientists discovered that the burden of defective mitochondria in mothers increases with age. “Unfortunately, the bad mitochondria that build up in mothers as they get older is passed down to their children,” Rothman said.
However, the good news is that it was possible to reduce both accumulation and inheritance of the defective mitochondria: the researchers found that a single gene change that makes the animals age more slowly and that extends their lifespan mitigates these problems.
“Slowing the ‘aging clock’ appears to cause defective mitochondria to accumulate more slowly, raising the possibility that anti-aging interventions could result in healthier mitochondria,” noted Rothman, who is also the founding Director of the Center for Aging and Longevity at UCSB. The research was funded in part by the National Institute on Aging.
These discoveries point to future strategies for removing debilitated mitochondria and rejuvenating cells, paving the way toward additional years of vibrant, disease-free life for all of us to enjoy.
Photo Credits and News Source: University of California - Santa Barbara.
World Mitochondria Society
Annual World Congress on Targeting Mitochondria
LinkedIn | Facebook
Inflammation-Triggered Mitochondrial and Metabolic Disruptions Steering the Transition from Acute to Chronic Pain
Niels Eijkelkamp and his team from the University Medical Center Utrecht have unveiled the enigmatic reasons behind lingering pain after inflammation, marking a significant breakthrough in chronic pain research.
-
Mitochondrial Disruptions: Persistent mitochondrial and metabolic disturbances in sensory neurons post-inflammation are identified as major contributors to enduring pain.
-
Redox Imbalance Impact: Disturbed redox balance in dorsal root ganglion (DRG) cells is linked to the failure of resolving inflammatory pain.
-
ATPSc-KMT Connection: Increased expression of the mitochondrial protein ATPSc-KMT correlates with hyperalgesic priming, exacerbating disruptions in sensory neurons.
-
Restoration Possibilities: Inhibiting mitochondrial respiration, ATPSCKMT knockdown, or targeted metabolite supplementation shows promise in restoring inflammatory pain resolution, preventing chronic pain.
This groundbreaking study challenges traditional perspectives, highlighting inflammation-induced mitochondrial-dependent disturbances as key players in chronic pain development. The findings pave the way for targeted interventions, revolutionizing chronic pain management.
Artificial DNA Successfully Transcribed by Natural Enzyme, Paving the Way for Genetic Advancements
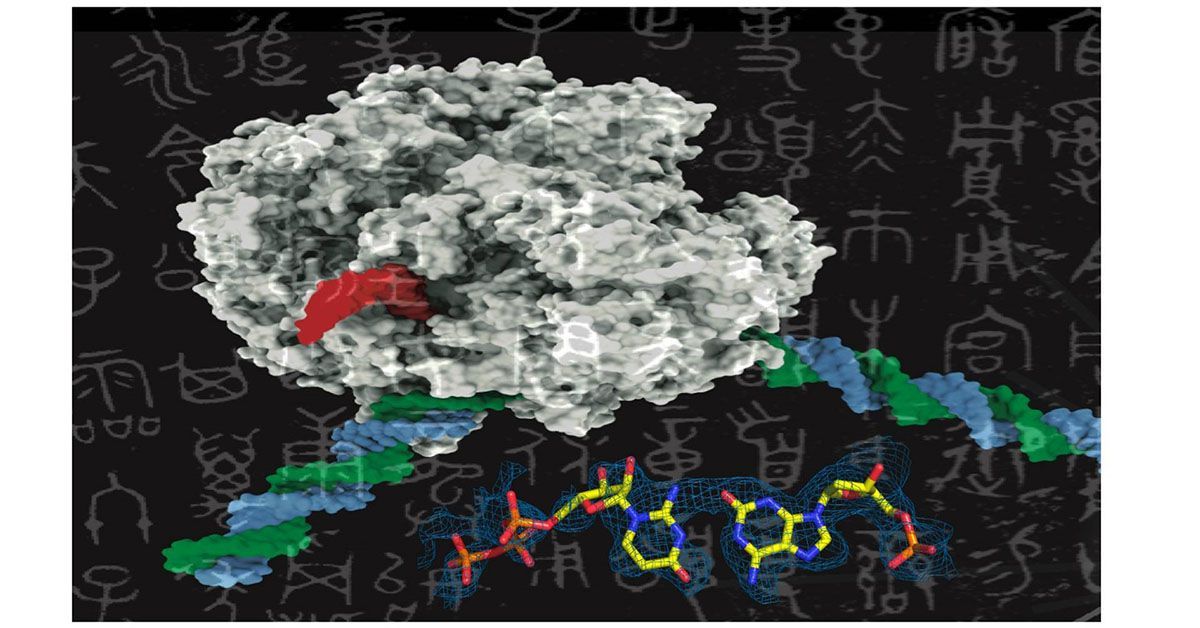
Like adding new letters to an existing language’s alphabet to expand its vocabulary, adding new synthetic nucleotides to the genetic alphabet could expand the possibilities of synthetic biology. This image shows a rendering of RNA polymerase (center) and a synthetic nucleotide (lower right).
Scientists at UC San Diego are exploring the expansion of the genetic alphabet, akin to introducing new letters to a language, by investigating the feasibility of incorporating synthetic nucleotides into the traditional four-nucleotide genetic alphabet (A, T, G, C). The findings were published in Nature Communications on December 12, 2023.
The application and risks of creating artificial DNA that can be transcribed by natural enzymes are as follows:
Applications:
1. New Medicinal Compounds: The ability to integrate synthetic nucleotides into DNA can lead to the creation of novel proteins, potentially resulting in new drugs and therapeutic agents.
2. Advanced Genetic Research: This technology can facilitate deeper understanding of genetic processes and molecular biology, opening new avenues in genetic research.
3. Synthetic Biology Innovations: Expanding the genetic code can lead to the development of new biological systems and organisms with customized features or abilities.
4. Biotechnology Advancements: It could enable the creation of more efficient biofuels, enhanced agricultural products, or new materials through synthetic biology.
5. Understanding Extraterrestrial Life: The research, initially supported by NASA, could provide insights into how extraterrestrial life might develop or be structured.
Risks:
1. Unintended Genetic Consequences: Introducing synthetic elements into the genetic code could have unforeseen effects on genetic stability, gene expression, or cellular function.
2. Biosecurity Concerns: There's a risk that this technology could be misused to create harmful organisms or biological agents.
3. Ethical and Regulatory Challenges: Manipulating the genetic code raises ethical questions about the extent of human intervention in natural processes and the need for robust regulatory frameworks.
4. Environmental Impact: If synthetic organisms were to be released into the environment, they could potentially disrupt ecosystems or outcompete natural species.
5. Technical Limitations and Errors: The precision required in this technology is high, and any errors in the transcription process could lead to detrimental effects or failed experiments.
Prof. Marvin Edeas, chairman of the scientific committee commented: "While the development of artificial DNA offers exciting potential for advancements in medicine and biotechnology, it also poses significant ethical, environmental, and biosecurity challenges that need to be carefully managed."
Photo Credits: UC San Diego Health Sciences
More Articles...
- Study Discovers Single-Celled Gut Protists Flourishing Without Mitochondria
- Blood Tests Uncover Biomarkers Linked to Suicidal Thoughts
- Mitochondrial Inhibitors Extend Lifespan in C. elegans: Insights from a Longevity Study
- Decoding Chronic Fatigue: The Crucial Role of WASF3 in Mitochondrial Dysfunction in ME/CFS




























































