Pseudomonas Bacteria Disrupt Mitochondrial Energy Production to Evade Immune Response
New research reveals how Pseudomonas aeruginosa dampens macrophage bioenergetics by targeting mitochondrial pathways, opening doors for novel therapeutic approaches.
December 7, 2025
The bacterium Pseudomonas aeruginosa, commonly found in freshwater, hot tubs, and pools, poses a serious threat when it infects humans. Known for causing severe infections, particularly in immunocompromised individuals or burn patients, this opportunistic pathogen has evolved resistance to multiple antibiotics, making treatment increasingly difficult.
In a recent study published in eLife, Harvard researchers Laurence Rahme and Arijit Chakraborty uncovered a mechanism by which P. aeruginosa suppresses immune responses. They found that the bacterium produces a chemical, 2-aminoacetophenone (2-AA), that interferes with mitochondrial energy production in macrophages, crippling their ability to fight infections.
How 2-AA Targets Mitochondrial Energy Production
Macrophages, essential components of the innate immune system, rely heavily on energy generated by their mitochondria to engulf and eliminate pathogens. However, the presence of 2-AA significantly reduces adenosine triphosphate (ATP) levels in these cells, disrupting their bioenergetics.
ATP, the cell’s energy currency, is primarily produced through two pathways: glycolysis in the cytoplasm and oxidative phosphorylation in the mitochondria. While glycolysis yields a modest 2 ATP molecules per glucose, mitochondrial oxidative phosphorylation generates approximately 30 ATP molecules per glucose, making it the most efficient energy source.
Using Seahorse assay technology, the researchers measured oxygen consumption—a hallmark of oxidative phosphorylation—and observed a significant drop in cells exposed to 2-AA. This indicated that 2-AA specifically targets mitochondrial energy production, effectively shutting down the more efficient ATP-generating pathway.
Additionally, 2-AA was found to block pyruvate transport into mitochondria, leaving excess pyruvate in the cytoplasm. Pyruvate is a critical metabolite that fuels the Krebs cycle and oxidative phosphorylation within mitochondria. Without it, the energy output from macrophage mitochondria is severely impaired, leading to diminished immune responses.
Mitochondrial Dysfunction in Living Systems
To validate these findings, Rahme and her team conducted experiments in mice infected with either wild-type P. aeruginosa or a mutant strain unable to produce 2-AA. In mice infected with the wild-type bacteria, ATP levels in the spleen—a key organ for immune responses—dropped within 24 hours, while those infected with the mutant strain maintained normal energy levels.
Further analysis revealed reduced levels of acetyl-cofactor A, a mitochondrial metabolite derived from pyruvate, in wild-type infections. This confirmed that 2-AA disrupts mitochondrial bioenergetics. Importantly, the absence of 2-AA allowed macrophages to combat bacterial infections more effectively, leading to a lower bacterial burden by day 10.
Implications for Mitochondria-Targeted Therapies
As antibiotic resistance continues to rise, targeting host-pathogen interactions presents a promising therapeutic strategy. Kayeen Vadakkan, a microbiologist at St. Mary’s College, Thrissur, who was not involved in the study, highlighted the potential of targeting 2-AA to enhance mitochondrial function in macrophages. “We can complement our immune system,” Vadakkan said, suggesting that blocking 2-AA could restore macrophage energy production and improve their ability to fight infections.
Rahme’s laboratory is developing inhibitors of MvfR, a transcription factor necessary for 2-AA production. Early results show promise, but further studies are needed to evaluate their clinical safety and efficacy.
Beyond infection, 2-AA’s ability to suppress mitochondrial activity and reduce inflammation hints at its potential application in treating autoimmune diseases like rheumatoid arthritis and lupus, where overactive macrophages drive inflammation.12 “2-AA is a molecule which is anti-inflammatory in nature,” Chakraborty noted, emphasizing its dual therapeutic potential.
Bridge Over Troubled Cells: Bone Marrow Stromal Cells Transfer Mitochondria to Boost T Cells
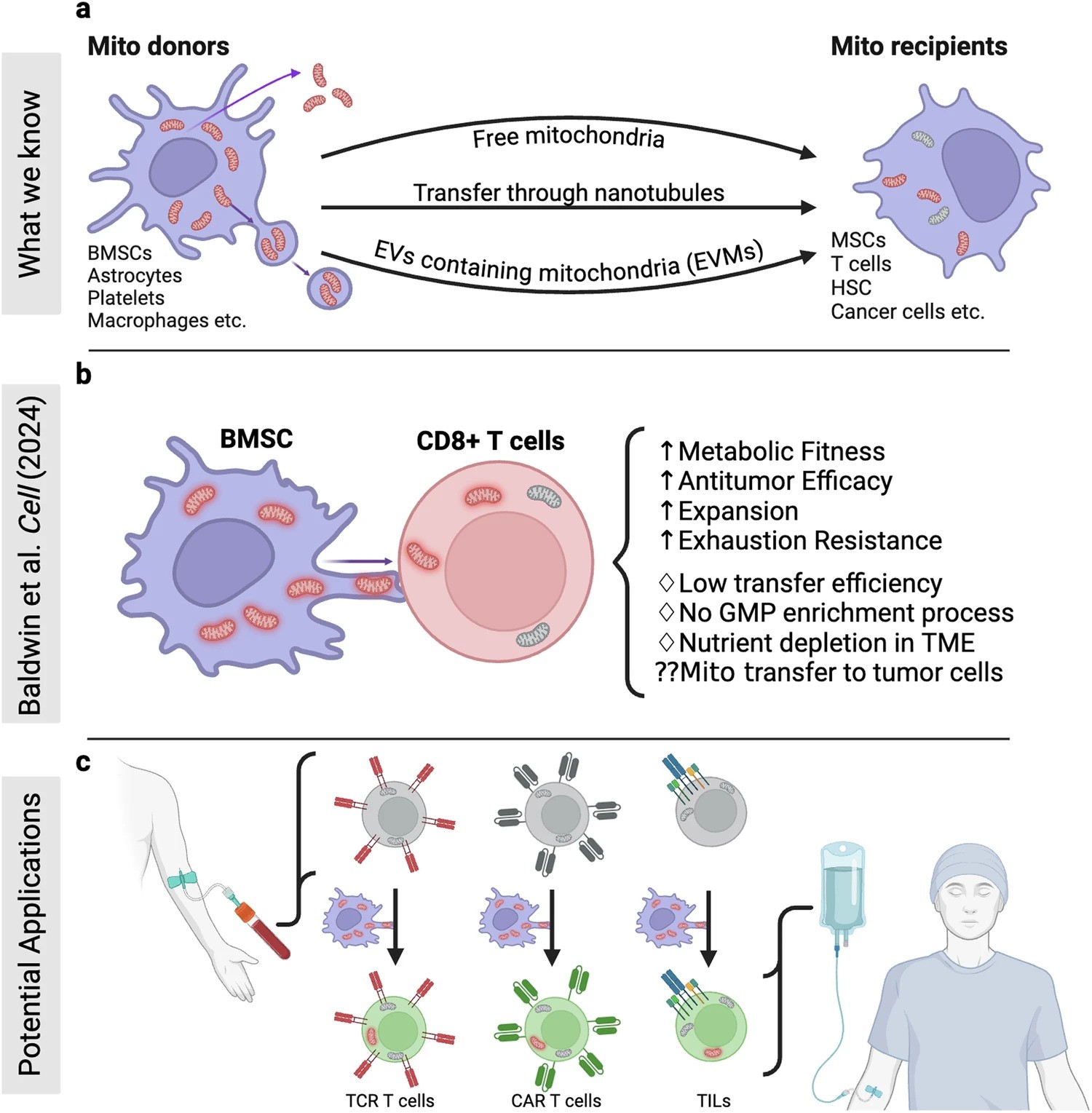
In a recent research published in Signal Transduction and Targeted Therapy, Lars Fabian Prinz,and his team present an innovative approach to combat T cell exhaustion using a bone marrow stromal cell (BMSC)-based mitochondrial transfer platform. This technique could revolutionize adoptive T cell therapies for cancer patients by addressing mitochondrial dysfunction in T cells.
- Intercellular Mitochondrial Transfer: BMSCs transfer functional mitochondria to CD8+ T cells via tunneling nanotubes (TNTs), enhancing T cell metabolic fitness.
- Improved Anti-Tumor Activity: Transferred mitochondria significantly boosted T cell resistance to exhaustion and anti-tumor effectiveness, both in vitro and in vivo.
- Enhanced Therapeutic Potential: Mitochondrial-enriched (Mito+) T cells exhibited increased proliferation, reduced apoptosis, and higher cytotoxicity in tumor environments.
The study demonstrated enhanced outcomes in mouse models of melanoma and leukemia, with Mito+ T cells showing improved tumor suppression and survival rates. Challenges remain, including optimizing transfer efficiencies and overcoming tumor microenvironment constraints. However, this discovery opens doors for both cell-based and systemic therapies to modulate mitochondrial transfer for cancer treatment.
Figure Description
a Building on mitochondrial transfer techniques described in the literature, Baldwin and colleagues introduce a method to fortify CD8+ T cells with mitochondria transferred through tunneling nanotubules from bone marrow stromal cells (BMSCs). b The transfer results in T cells being more resistant against exhaustion and having higher anti-tumor activity in-vitro and in-vivo. c This could be applied to improve adoptive T cell therapies to treat patients with cancer. (Created with BioRender.com)
Source: https://www.nature.com/articles/s41392-024-02079-6
Image Credits: Prinz, L.F., Ullrich, R.T. & Chmielewski, M.M. B Sig Transduct Target Ther (2024).
Study Shows Decreased Mitochondrial Creatine Kinase Impairs Muscle Function Independently of Insulin in Type 2 Diabetes
Published in Science Translational Medicine
Researchers from Karolinska Institutet have uncovered a key factor contributing to impaired muscle energy production in individuals with type 2 diabetes. The study reveals that people with type 2 diabetes have reduced levels of creatine kinase, a protein responsible for metabolizing and converting creatine in muscle cells. This deficiency hampers mitochondrial function—the "powerhouses" of cells—leading to decreased energy production and increased cellular stress.
Creatine, a compound naturally produced by the body and found in foods like meat and fish, is critical for muscle function. Although creatine supplementation is popular for enhancing exercise performance, elevated blood creatine levels have been linked to an increased risk of type 2 diabetes. This study demonstrates that the reduced levels of creatine kinase observed in people with type 2 diabetes lead to impaired creatine metabolism, explaining the accumulation of creatine in their bloodstream.
“Our findings suggest that impaired creatine metabolism is a consequence of type 2 diabetes rather than a cause,” says Professor Anna Krook from the Department of Physiology and Pharmacology at Karolinska Institutet, the study’s principal investigator.
Moreover, the study shows that reduced creatine kinase levels directly affect mitochondrial function, independent of creatine availability. This discovery highlights the multifaceted role of creatine kinase in cellular energy production.
“This is consistent with the poorer energy metabolism seen in people with type 2 diabetes,” adds Professor Krook. “In the future, regulating creatine kinase could be explored as a potential treatment strategy for metabolic diseases like obesity and diabetes.”
The next phase of research will focus on identifying the molecular mechanisms linking creatine kinase levels to mitochondrial function.
Mitochondrial Dysfunction Disrupts Gut Microbiome, Possible Trigger for Crohn's Disease
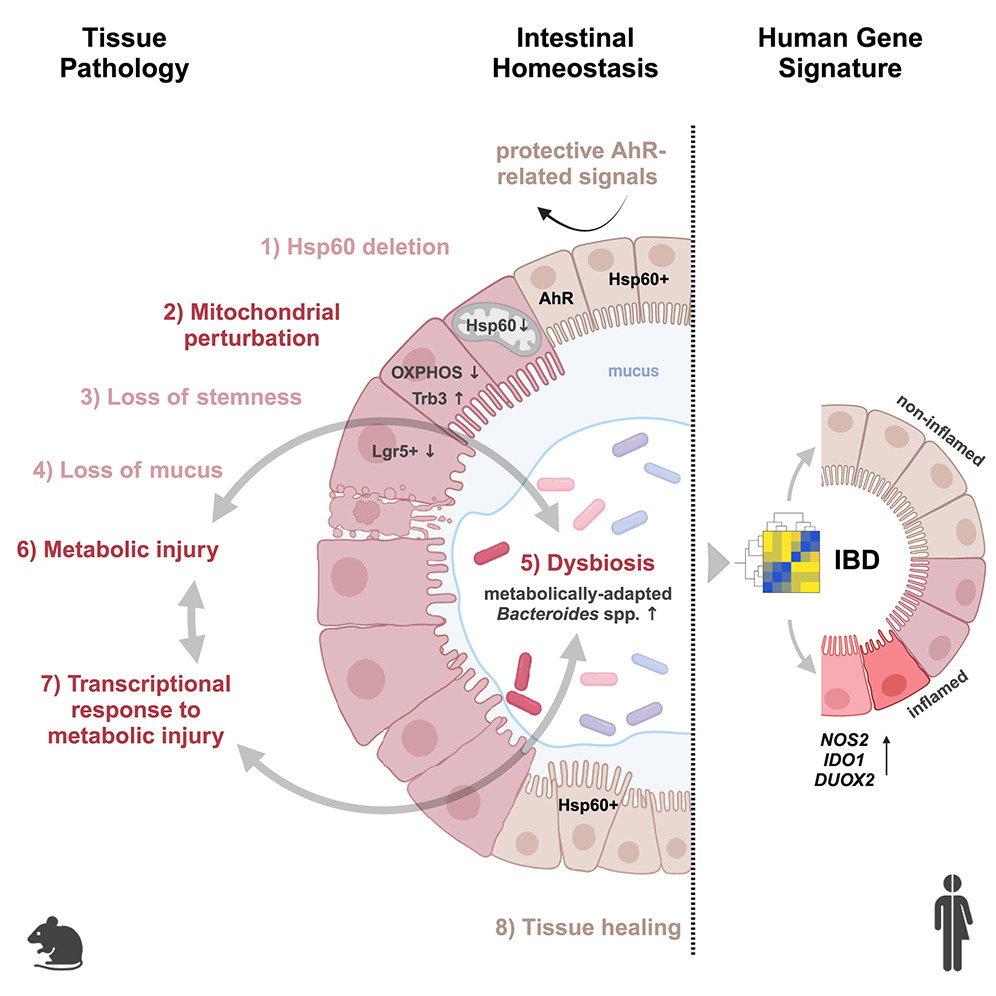
A recent study led by Prof. Dr. Dirk Haller at the Technical University of Munich (TUM) uncovers a critical connection between mitochondrial dysfunction and Crohn's disease (CD). The research demonstrates that defective mitochondria cause intestinal epithelial damage, triggering significant changes in the gut microbiome—key factors in the onset of this chronic inflammatory condition.
Key Findings:
- Mitochondrial dysfunction leads to tissue damage in the intestinal epithelium, mimicking Crohn's disease symptoms.
- Disruptions in mitochondrial function result in alterations to the gut microbiome composition.
- This marks the first demonstration of a causal link between mitochondrial health and gut inflammation.
Implications for Treatment: Current Crohn's disease therapies focus on symptom management, but these findings open the door to novel approaches that target mitochondrial repair, potentially offering more effective, long-term solutions for managing CD.
© Photo Credits: Urbauer, Elisabeth et al. Cell Host & Microbe (2024)
Mitochondria’s Secret Strategy: How Cells Survive Starvation

Mitochondria, often called the “powerhouses” of cells, are responsible for producing energy and vital molecules that cells need to function. However, until now, scientists have puzzled over how mitochondria manage to sustain these processes when cells are starved of nutrients. Researchers have uncovered that in low-resource conditions, mitochondria adopt a surprising strategy: they divide into two specialized forms. One form focuses on energy production to keep the cell powered, while the other concentrates on creating essential building blocks needed for repair and growth. This division of labor allows cells to survive and adapt even in challenging environments.
This remarkable discovery may also shed light on how some cancers thrive in hostile conditions within the body. Certain cancer cells appear to use the same mitochondrial strategy to fuel their growth and survival when nutrients are scarce. By splitting their functions, mitochondria in these cancer cells ensure the production of energy and key cellular components, enabling tumors to persist and grow even in environments that should inhibit them. Understanding this process offers new insights into cancer resilience and could pave the way for innovative treatments targeting mitochondrial functions in cancer cells.
This breakthrough highlights the extraordinary adaptability of mitochondria, offering a deeper understanding of cellular survival and disease mechanisms.
© World Mitochondria Society (WMS)




























































