Dnmt3a mutation enhances mitochondrial function in blood stem cells, leading to clonal expansion and increased disease risk

The Jackson Laboratory’s recent study, published on April 16, 2025, in Nature Communications, uncovers how a common age-related mutation in the Dnmt3a gene enhances mitochondrial function in blood stem cells, leading to clonal hematopoiesis—a condition that increases the risk of heart disease, blood cancers, and other illnesses.
Key Findings
- Dnmt3a Mutation and Mitochondrial Enhancement: The Dnmt3a mutation boosts mitochondrial energy production in blood stem cells, granting them a self-renewal advantage.
- Development of Clonal Hematopoiesis: This advantage leads to clonal hematopoiesis, where mutated stem cells dominate, increasing the risk of various diseases.
- Prevalence in Aging Population: Clonal hematopoiesis is common in the elderly, affecting over half of individuals aged 80 and above.
- Inflammatory Impact: Mutated stem cells produce inflammatory molecules that disrupt blood production and weaken the immune system.
- Therapeutic Potential: The study suggests that targeting the enhanced mitochondrial function could be a strategy to prevent or treat clonal hematopoiesis.
These findings show that Dnmt3a-mutated cells rely on enhanced mitochondrial function to dominate,” said senior author Dr. Jennifer Trowbridge. “Targeting this metabolic vulnerability could be a promising strategy to prevent or treat clonal hematopoiesis and its downstream effects.
Marvin Edeas, INSERM, University of Paris, co-organiser of the Targeting Longevity & Mitochondria congress, stated, “This discovery brings new insight into how the aging process is closely linked to energy production in our cells, especially through mitochondria, the cell’s powerhouses. When mitochondria become overactive due to certain mutations, they give some cells an unfair growth advantage, which can lead to harmful conditions like clonal hematopoiesis and increase the risk of diseases such as blood cancer or heart problems. By targeting and calming this mitochondrial overactivity, we may be able to stop these abnormal cells from expanding and protect against age-related diseases. This could represent an important step toward healthier aging.
In a separate publication in Nature published April 16th, Dr. Jennifer Trowbridge provided preclinical rationale for investigating metformin as a preventive intervention against DNMT3A R882 mutation-driven clonal haematopoiesis in humans.
What is DNMT3A?
- It is de novo methyltransferase, meaning it can establish new methylation patterns during development and in adult tissues.
- It plays a crucial role in stem cell differentiation, development, and maintaining genomic stability.
- Mutations in Dnmt3a are commonly found in clonal hematopoiesis, leukemias, and other age-related diseases.
- Recent research shows these mutations can alter mitochondrial function, giving mutant cells a growth advantage.
Zika Virus NS1 Hijacks Mitochondria Through Tunneling Nanotubes for Immune Evasion
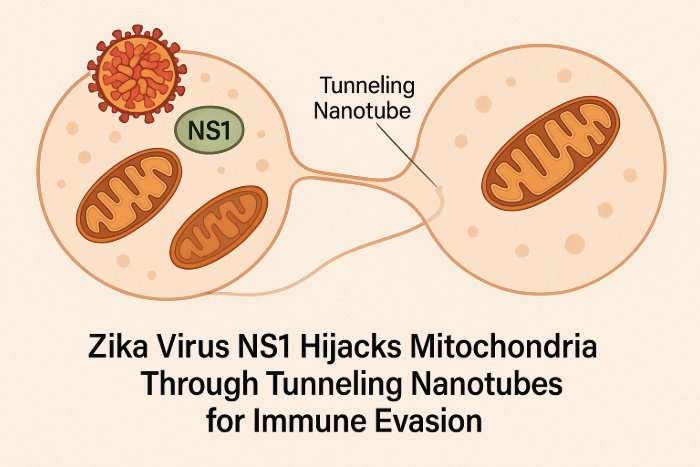
The study titled “Zika virus NS1 drives tunneling nanotube formation for mitochondrial transfer and stealth transmission in trophoblasts” (Nature Communications, February 2025), published by Dr. Indira Mysorekar and her team, explores how Zika virus (ZIKV) utilizes a stealth mechanism to spread among placental trophoblasts. The research focuses on the role of the viral protein NS1 in facilitating this process.
Key Findings:
- Tunneling Nanotube Formation: ZIKV induces the formation of tunneling nanotubes (TNTs) in placental trophoblasts, which serve as conduits for transferring viral components and mitochondria to neighboring uninfected cells.
- Role of NS1 Protein: The non-structural protein 1 (NS1) of ZIKV, particularly its N-terminal 1–50 amino acids, is critical for triggering TNT formation in host cells.
- Immune Evasion: Trophoblasts infected with a TNT-deficient ZIKV mutant elicited a stronger antiviral interferon response compared to those infected with wild-type ZIKV, suggesting that TNT-mediated transmission helps the virus evade host immune defenses.
- Mitochondrial Hijacking: ZIKV infection or NS1 expression leads to increased mitochondrial levels in trophoblasts, with mitochondria being transferred via TNTs from healthy to infected cells, potentially supporting viral replication and survival.
These findings shed light on a novel mechanism by which ZIKV spreads and evades the immune system, offering potential targets for therapeutic intervention.
Revolutionary Breakthrough in Mitochondrial Production Offers Hope for Degenerative Diseases

Scientists have achieved a groundbreaking advancement in the mass production of high-quality human mitochondria, paving the way for transformative treatments in degenerative diseases. By refining stem cell culture conditions, researchers have successfully increased mitochondrial production by an astonishing 854-fold while significantly enhancing their energy output.
Key Highlights:
- Innovative Method: The new method leverages human mesenchymal stem cells and a specially designed culture medium called "mito-condition." This medium integrates nine essential components, including growth factors and human platelet lysate, to optimize mitochondrial production.
- Enhanced Performance: The engineered mitochondria exhibit remarkable therapeutic benefits, notably accelerating cartilage regeneration in osteoarthritis models. They produce 5.7 times more ATP than naturally occurring mitochondria and maintain stable performance even post-isolation.
- Clinical Implications: This breakthrough addresses the long-standing bottleneck in mitochondrial transplantation, which has been constrained by limited supply and inconsistent quality. The ability to produce high-quality mitochondria on a large scale opens new avenues for treating a wide spectrum of mitochondrial dysfunction-related diseases, from joint degeneration to cardiovascular disorders.
- Mechanistic Insights: The study reveals how cells can be reprogrammed to prioritize mitochondrial synthesis by activating the AMPK pathway, a crucial cellular energy sensor. This process downregulates energy-intensive activities like autophagy and secretion, effectively boosting mitochondrial biogenesis.
- Future Applications: Beyond osteoarthritis, this technology holds promise for conditions such as heart disease, neurodegenerative disorders, and wound healing. The concept of organelle tuning could also be adapted to generate other cellular components, broadening the horizons of cell engineering and therapeutic applications.
Publication Details: DOI: 10.1038/s41413-025-00411-6 | Image @WMS
During Targeting Mitochondria 2025, this hot topic will be presented and discussed strongly.
How Mitochondria Organize Their Powerhouse Machinery for Optimal Performance

The study titled “In-cell architecture of the mitochondrial respiratory chain,” published in Science on March 20, 2025, presents significant advancements in understanding mitochondrial structure and function.
Key Highlights:
- In Situ Visualization: Researchers employed advanced imaging techniques to observe the mitochondrial respiratory chain within intact cells, providing a detailed view of its native architecture.
- Respiratory Supercomplexes: The study offers insights into how respiratory complexes assemble into supercomplexes, which are crucial for efficient electron transport and energy production in cells.
- Functional Implications: Understanding the organization of these supercomplexes sheds light on their role in cellular metabolism and energy conversion, potentially informing research into mitochondrial-related diseases.
Perspective:
- Challenging previous assumptions: The findings challenge long-standing models that assumed a more fluid, random distribution of respiratory chain components in mitochondrial membranes.
- Biological relevance: By analyzing structures in situ, this study underscores the importance of studying macromolecular organization in native cellular contexts, rather than relying only on purified proteins.
- Broader implications: These insights are critical not only for basic mitochondrial biology but also for understanding mitochondrial dysfunction in aging, neurodegenerative diseases, and metabolic disorders.
- New model for mitochondrial function: This study supports a model in which the geometrical and biochemical compartmentalization within cristae contributes significantly to the efficiency of oxidative phosphorylation.
These findings enhance our comprehension of mitochondrial function and may have implications for addressing metabolic disorders linked to mitochondrial dysfunction.
Why Our Cells Slow Down with Age: The Mitochondria Mystery
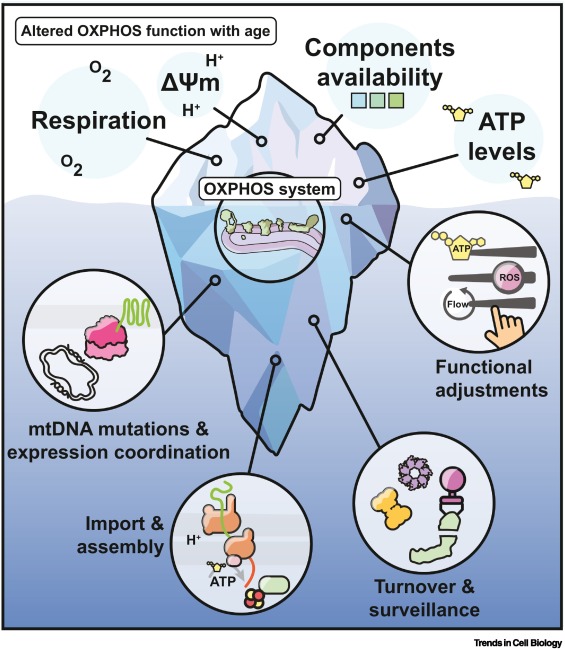
As we get older, our bodies slow down—but have you ever wondered why? New research points to tiny structures inside our cells called mitochondria. These are often called the “powerhouses” of the cell because they produce the energy we need to live. But with age, these powerhouses stop working as well as they used to.
Recent publication by Hanna Salmonowicz et al, have discovered that a key part of the energy-making system, known as OXPHOS, starts to break down over time. This affects how much energy our cells can produce, especially in important organs like the brain, heart, and muscles. It may also explain why we become more vulnerable to diseases as we age.
Main Points:
- Mitochondria need instructions from two sources - our cell’s nucleus and their own DNA—to work properly. With age, this teamwork gets disrupted.
- Mistakes build up in mitochondrial DNA, leading to damage in the energy system.
- Our cells struggle to bring in and assemble the proteins needed to keep mitochondria running.
- Damaged mitochondria make more harmful molecules called ROS (reactive oxygen species), which can hurt the cell even more.
- The effects of these changes are especially strong in parts of the body that use a lot of energy, like the brain and heart.
Conclusion:
This research helps explain why we feel more tired, weaker, and more prone to disease as we age. But it also brings hope. With new tools and technology, scientists are finding ways to better understand and possibly fix these tiny energy factories. Keeping our mitochondria healthy could be the key to living longer, stronger, and healthier lives.




























































