Tiny Messengers, Big Impact: Exosomes as Gatekeepers of Mitochondrial Function
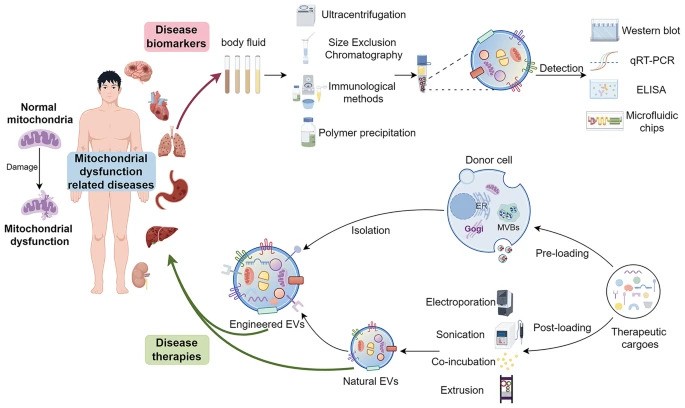
The article titled “Extracellular vesicles: opening up a new perspective for the diagnosis and treatment of mitochondrial dysfunction” was published in the Journal of Nanobiotechnology on August 14, 2024.
Key Points:
- Mitochondrial Dysfunction: Mitochondria are essential for energy production in eukaryotic cells. Disruptions such as oxidative stress, calcium imbalances, and mitochondrial DNA abnormalities can lead to mitochondrial dysfunction, contributing to various diseases.
- Extracellular Vesicles (EVs): EVs are cell-derived nanovesicles that facilitate intercellular communication by transporting proteins, lipids, and nucleic acids. They are categorized based on size into small EVs (sEVs, <200 nm) and large EVs (lEVs, >200 nm).
- EVs and Mitochondrial Components: Certain EV subtypes are enriched with mitochondrial components, including intact mitochondria. These EVs can transfer mitochondrial elements to recipient cells, influencing mitochondrial function.
- Modulation of Mitochondrial Function: EVs can affect mitochondrial homeostasis, apoptosis, and reactive oxygen species (ROS) generation in target cells by delivering specific substances.
- Therapeutic Potential: Research is exploring the artificial modification of EVs as delivery vehicles for therapeutic agents targeting mitochondria, offering new avenues for treating conditions associated with mitochondrial dysfunction.
Perspective:
This review highlights the emerging role of EVs in modulating mitochondrial function, presenting innovative strategies for diagnosing and treating diseases linked to mitochondrial dysfunction. By leveraging the natural communication pathways of EVs, there is potential to develop targeted therapies that restore mitochondrial health, offering hope for conditions such as neurodegenerative diseases, cardiovascular disorders, and cancer. Future research should focus on understanding the mechanisms governing EV-mediated mitochondrial modulation and developing safe, effective EV-based therapeutic approaches.
Learn more in the full article.
© Image: Li, J., Wang, T., Hou, X. et al. J Nanobiotechnol22, 487 (2024).
Microglia Transfer Healthy Mitochondria to Rescue Neurons from Neurodegeneration
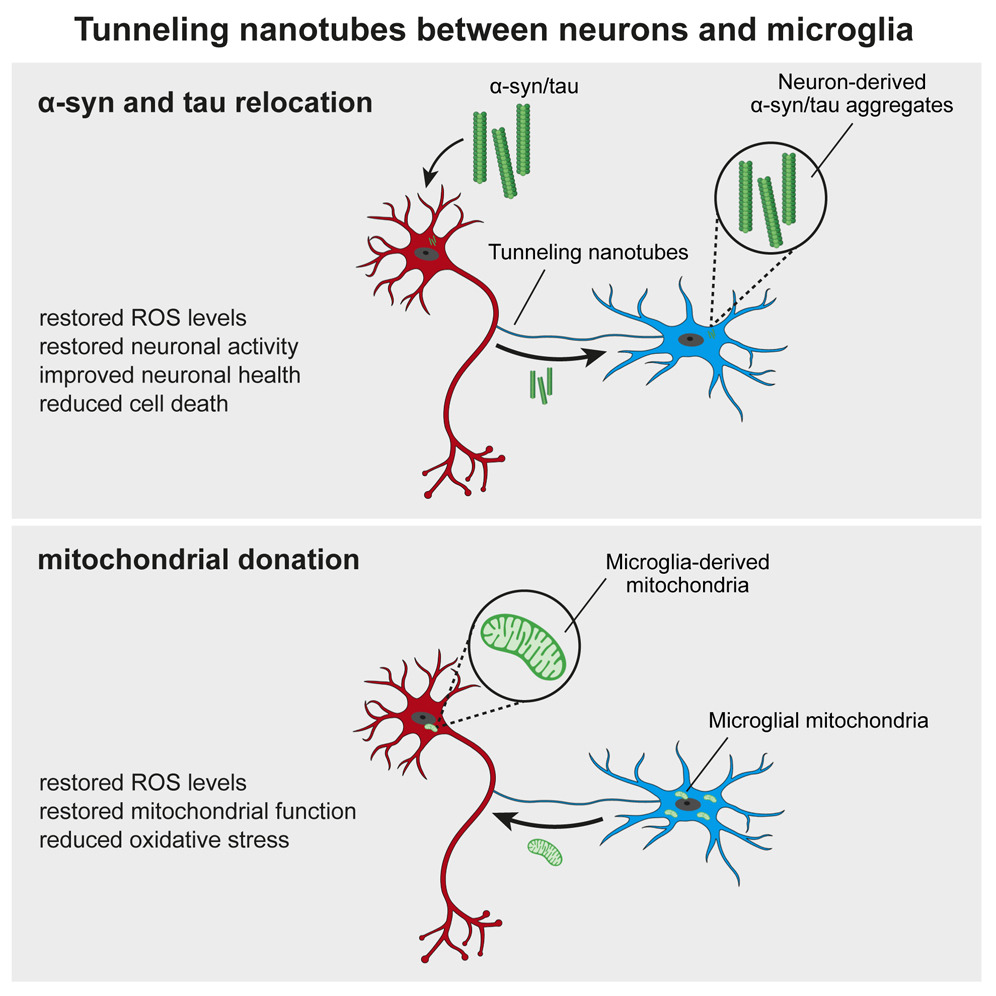
A new study led by Prof. Michael T. Heneka from the Luxembourg Centre for Systems Biomedicine, University of Luxembourg, uncovers a crucial neuroprotective role of microglia in mitochondrial health. Published in Neuron, the research reveals how microglia use tunneling nanotubes (TNTs) to transfer healthy mitochondria to stressed neurons, rescuing them from toxic protein aggregates linked to Parkinson’s and Alzheimer’s diseases.
TNTs act as direct cellular bridges, allowing microglia to exchange organelles, vesicles, and proteins with neurons. This process helps remove harmful alpha-synuclein (a-syn) and tau aggregates, reducing oxidative stress and restoring normal gene expression. However, mutations in LRRK2 (Gly2019Ser) and TREM2 (T66M, R47H) impair TNT-mediated mitochondrial transfer, potentially exacerbating neurodegeneration.
These findings highlight the vital role of mitochondrial quality control and microglia-neuron interactions in neuroprotection. Targeting TNT-mediated mitochondrial transfer could offer new therapeutic strategies for neurodegenerative diseases.
For more details, read the full study: DOI: 10.1016/j.neuron.2024.06.029.
© Image Credits: Scheiblich et al. Neuron, 2024
Targeting Mitophagy in Neurodegenerative Diseases: A Promising Therapeutic Avenue
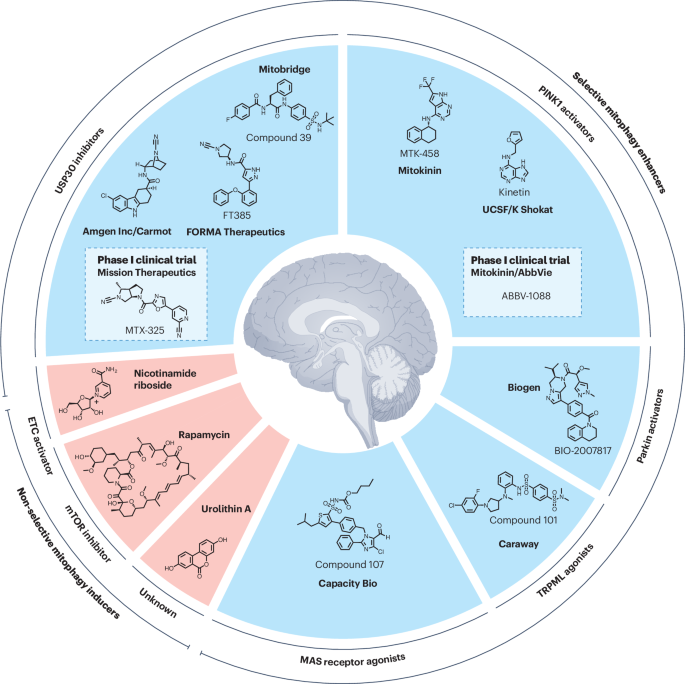
Mitochondrial dysfunction is a central feature of several neurodegenerative diseases, including Parkinson’s disease, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, and Huntington’s disease. A groundbreaking study published in Nature Reviews Drug Discovery by Odetta Antico, Paul W. Thompson, Nicholas T. Hertz, Miratul M. K. Muqit, and Laura E. Parton highlights the potential of targeting mitophagy — the cellular process that eliminates damaged mitochondria — as a therapeutic strategy for these debilitating conditions.
Mutations in genes related to mitophagy deficits are often linked to familial forms of Parkinson’s disease and ALS. Enhancing the mitophagy pathway could address this underlying pathogenic mechanism, providing neuroprotection and disease-modifying effects—an urgent unmet need in neurodegenerative disease management.
Excitingly, small molecules aimed at enhancing mitophagy, such as USP30 inhibitors and PINK1 activators, are now entering phase I clinical trials. This marks a pivotal moment in translating preclinical evidence into potential treatments that could revolutionize care for patients affected by these devastating diseases.
Figure Description: Selective and non-selective mitophagy activators.
Read the full article: Nature Reviews Drug Discovery (2025).
The future of cancer immunotherapy will come through mitochondria: Cancer cells hijack T cells
Cancer cells ‘poison’ the immune system with tainted mitochondria
A study reveals how cancer cells suppress immune responses by transferring mutated mitochondria to tumor-infiltrating T cells. Researchers identified mitochondrial DNA mutations in T cells that matched mutations in the cancer cells they infiltrated. This transfer impaired T cell metabolism and function, leading to senescence and a diminished ability to fight tumors.
The transfer occurs via tunneling nanotubes and extracellular vesicles, with cancer-derived mitochondria resisting normal degradation processes. These dysfunctional mitochondria disrupt T cell energy production, leaving the immune cells unable to mount effective antitumor responses.
The findings could explain why some patients fail to respond to immune checkpoint inhibitors like PD-1 blockers. Targeting this mitochondrial transfer mechanism might open new doors for the future of cancer immunotherapy.
A dedicated workshop on mitochondrial transfer and its implications for cancer therapy will be organized at Targeting Mitochondria 2025.
For full Article: https://www.nature.com/articles/d41586-025-00176-2
Ketone Bodies: A New Approach to Brain Function and Neurodegenerative Diseases
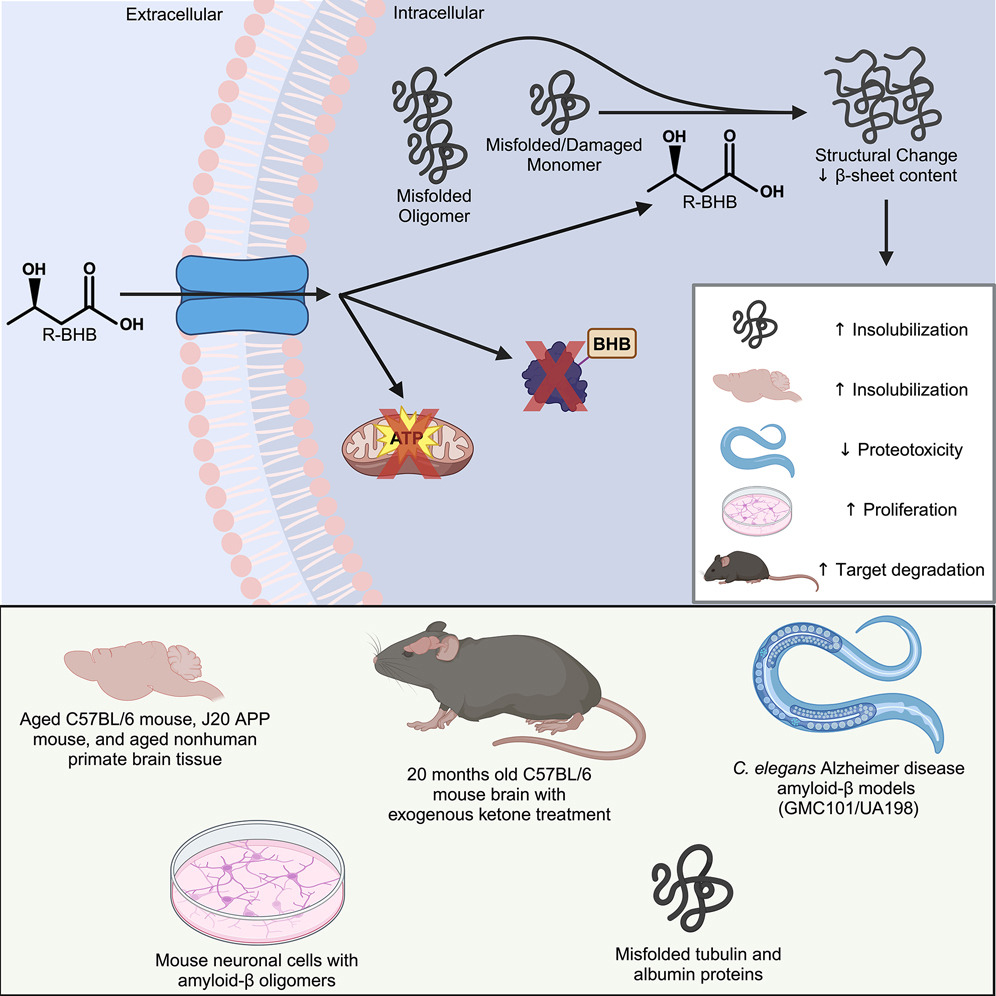
A recent study published in Cell Chemical Biology by researchers at the Buck Institute for Research on Aging has revealed intriguing new roles for ketone bodies, particularly β-hydroxybutyrate, in maintaining brain health. While ketone bodies are primarily recognized for their role in energy production, this research highlights their broader impact on mitochondrial function and protein homeostasis in the brain, offering promising insights for aging and neurodegenerative diseases like Alzheimer’s.
The study shows that β-hydroxybutyrate directly interacts with misfolded proteins in the brain, altering their structure and solubility to promote their clearance through autophagy. This process is crucial for supporting mitochondrial function, as it prevents the buildup of damaged proteins that can impair cellular health and energy production.
In addition, ketone bodies have a profound effect on protein quality control mechanisms within the brain. By influencing the proteome, ketones help enhance the clearance of dysfunctional proteins, ensuring that mitochondrial and cellular functions remain intact. This mechanism is vital for preserving the overall health of brain cells, especially as they age.
Experimental validation in animal models further supports these findings. When mice were fed ketone esters, the clearance of insoluble proteins was enhanced, preventing pathological aggregation. In nematodes expressing human amyloid beta, ketone treatment successfully reversed paralysis, suggesting a restoration of mitochondrial function and recovery from protein-induced damage.
These findings open up new potential therapeutic avenues for brain aging and neurodegenerative diseases. By manipulating ketone body levels, it may be possible to support mitochondrial function and facilitate the removal of damaged proteins from the brain. This approach could serve as a powerful strategy to mitigate the effects of aging on the brain and provide a novel way to treat neurodegenerative diseases.
The research underscores the emerging role of ketone bodies as signaling molecules that help regulate protein homeostasis and mitochondrial health in the brain. As mitochondrial dysfunction is a central factor in many neurodegenerative diseases, this study paves the way for new therapeutic approaches aimed at boosting mitochondrial resilience and improving brain health.
Image Credits: Sid Madhavan, Buck Institute for Research on Aging
More Articles...
- Pseudomonas Bacteria Disrupt Mitochondrial Energy Production to Evade Immune Response
- Bridge Over Troubled Cells: Bone Marrow Stromal Cells Transfer Mitochondria to Boost T Cells
- Mitochondrial Dysfunction Disrupts Gut Microbiome, Possible Trigger for Crohn's Disease
- Study Shows Decreased Mitochondrial Creatine Kinase Impairs Muscle Function Independently of Insulin in Type 2 Diabetes