Bridge Over Troubled Cells: Bone Marrow Stromal Cells Transfer Mitochondria to Boost T Cells
In a recent research published in Signal Transduction and Targeted Therapy, Lars Fabian Prinz,and his team present an innovative approach to combat T cell exhaustion using a bone marrow stromal cell (BMSC)-based mitochondrial transfer platform. This technique could revolutionize adoptive T cell therapies for cancer patients by addressing mitochondrial dysfunction in T cells.
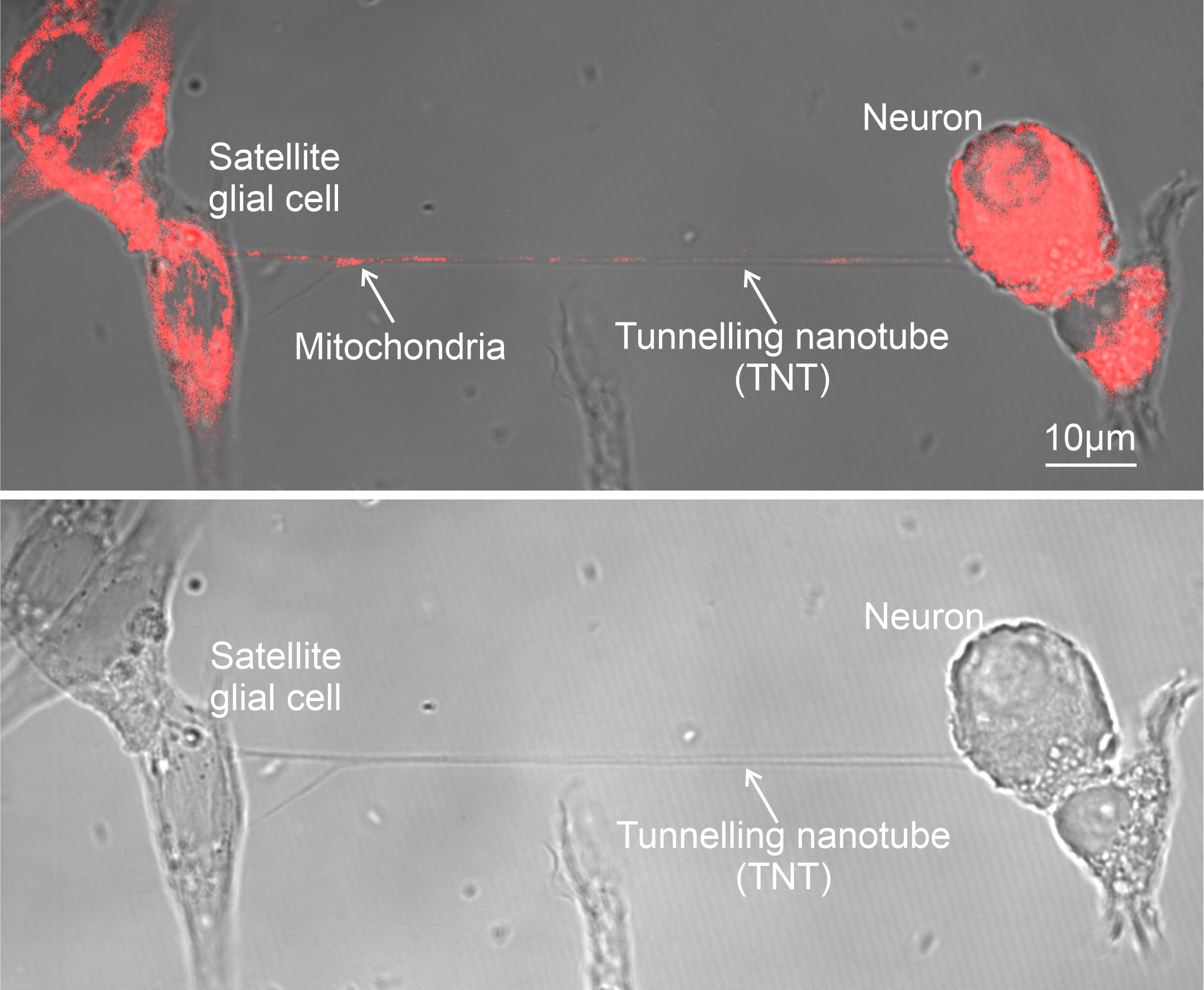
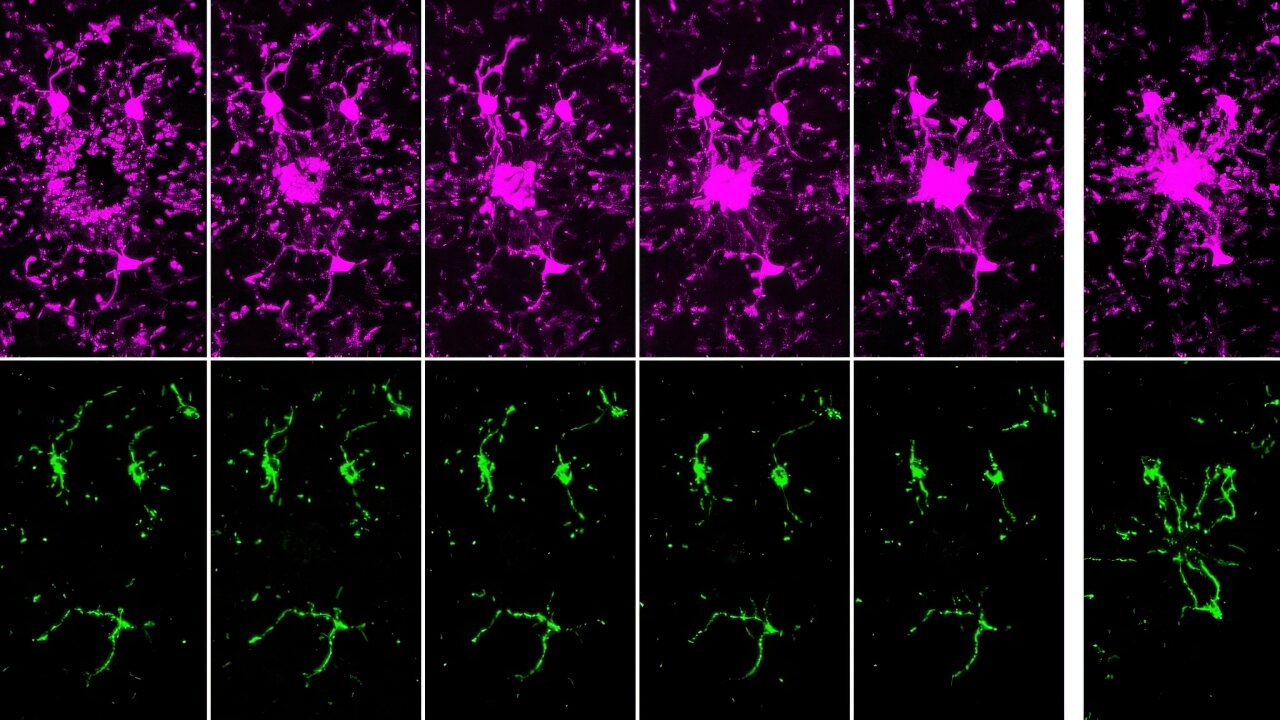
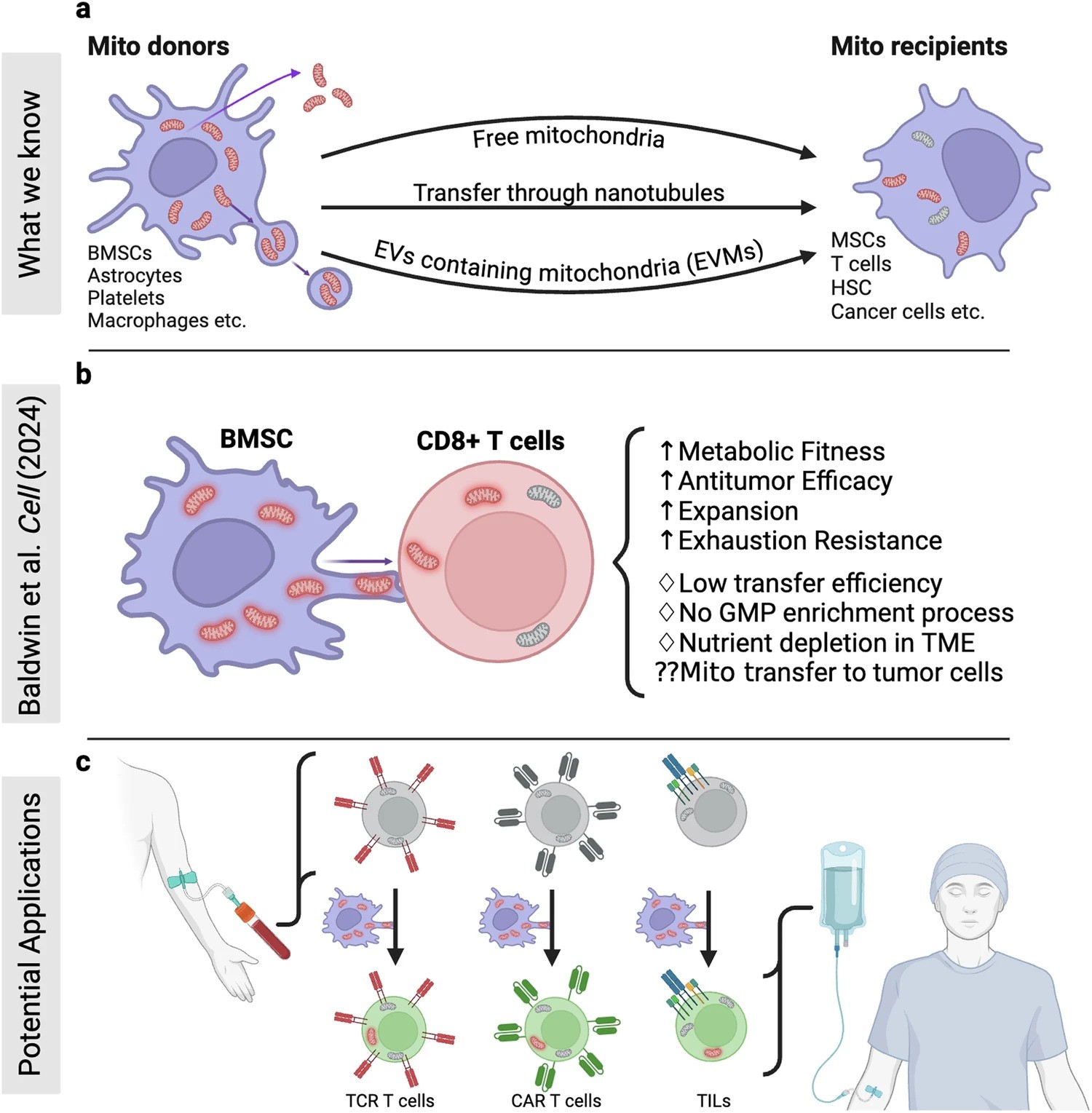
- Intercellular Mitochondrial Transfer: BMSCs transfer functional mitochondria to CD8+ T cells via tunneling nanotubes (TNTs), enhancing T cell metabolic fitness.
- Improved Anti-Tumor Activity: Transferred mitochondria significantly boosted T cell resistance to exhaustion and anti-tumor effectiveness, both in vitro and in vivo.
- Enhanced Therapeutic Potential: Mitochondrial-enriched (Mito+) T cells exhibited increased proliferation, reduced apoptosis, and higher cytotoxicity in tumor environments.
The study demonstrated enhanced outcomes in mouse models of melanoma and leukemia, with Mito+ T cells showing improved tumor suppression and survival rates. Challenges remain, including optimizing transfer efficiencies and overcoming tumor microenvironment constraints. However, this discovery opens doors for both cell-based and systemic therapies to modulate mitochondrial transfer for cancer treatment.
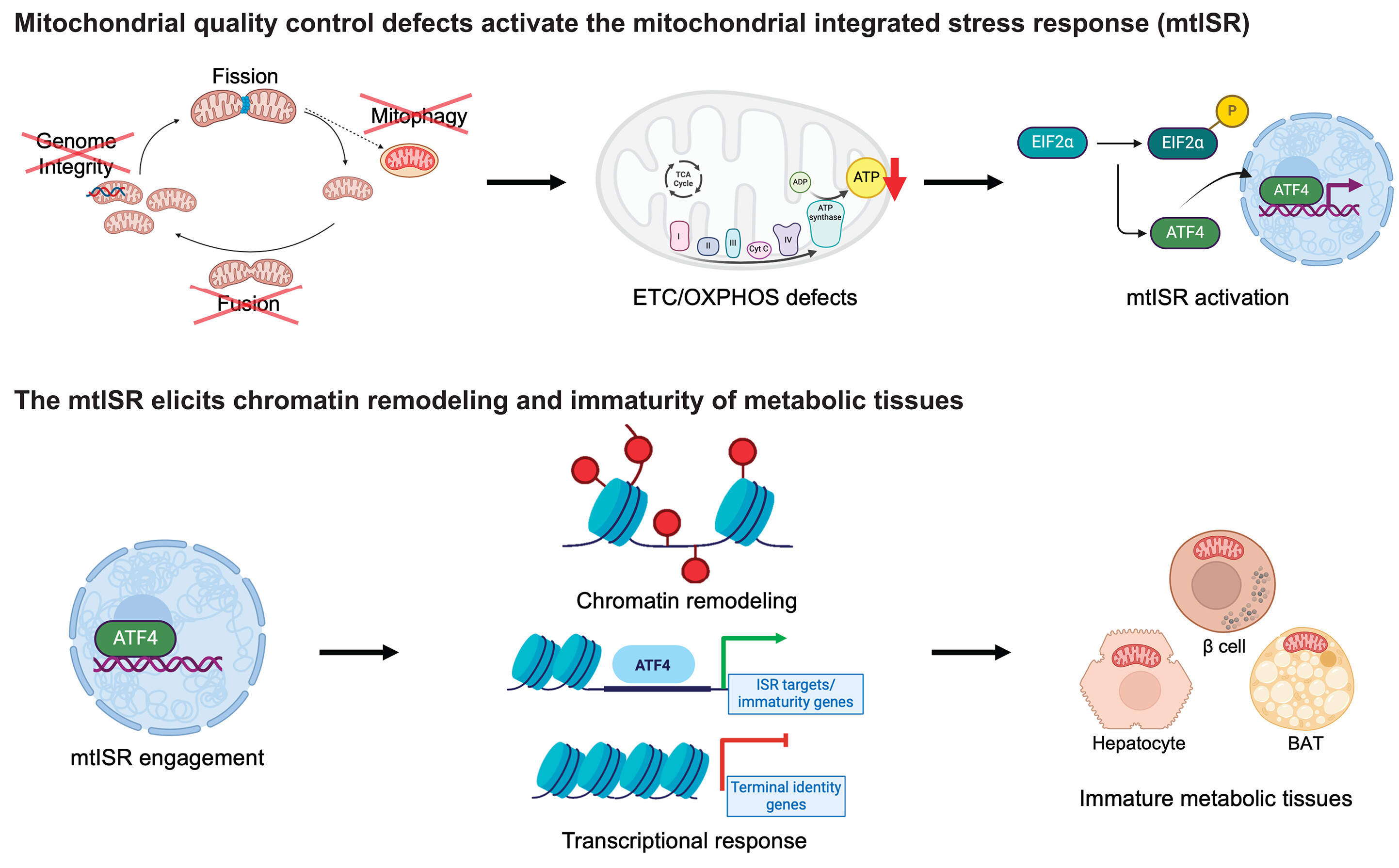
Figure Description
a Building on mitochondrial transfer techniques described in the literature, Baldwin and colleagues introduce a method to fortify CD8+ T cells with mitochondria transferred through tunneling nanotubules from bone marrow stromal cells (BMSCs). b The transfer results in T cells being more resistant against exhaustion and having higher anti-tumor activity in-vitro and in-vivo. c This could be applied to improve adoptive T cell therapies to treat patients with cancer. (Created with BioRender.com)
Source: https://www.nature.com/articles/s41392-024-02079-6
Image Credits: Prinz, L.F., Ullrich, R.T. & Chmielewski, M.M. B Sig Transduct Target Ther (2024).