Pseudomonas Bacteria Disrupt Mitochondrial Energy Production to Evade Immune Response
New research reveals how Pseudomonas aeruginosa dampens macrophage bioenergetics by targeting mitochondrial pathways, opening doors for novel therapeutic approaches.
December 7, 2025
The bacterium Pseudomonas aeruginosa, commonly found in freshwater, hot tubs, and pools, poses a serious threat when it infects humans. Known for causing severe infections, particularly in immunocompromised individuals or burn patients, this opportunistic pathogen has evolved resistance to multiple antibiotics, making treatment increasingly difficult.
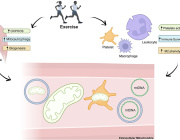
In a recent study published in eLife, Harvard researchers Laurence Rahme and Arijit Chakraborty uncovered a mechanism by which P. aeruginosa suppresses immune responses. They found that the bacterium produces a chemical, 2-aminoacetophenone (2-AA), that interferes with mitochondrial energy production in macrophages, crippling their ability to fight infections.
How 2-AA Targets Mitochondrial Energy Production
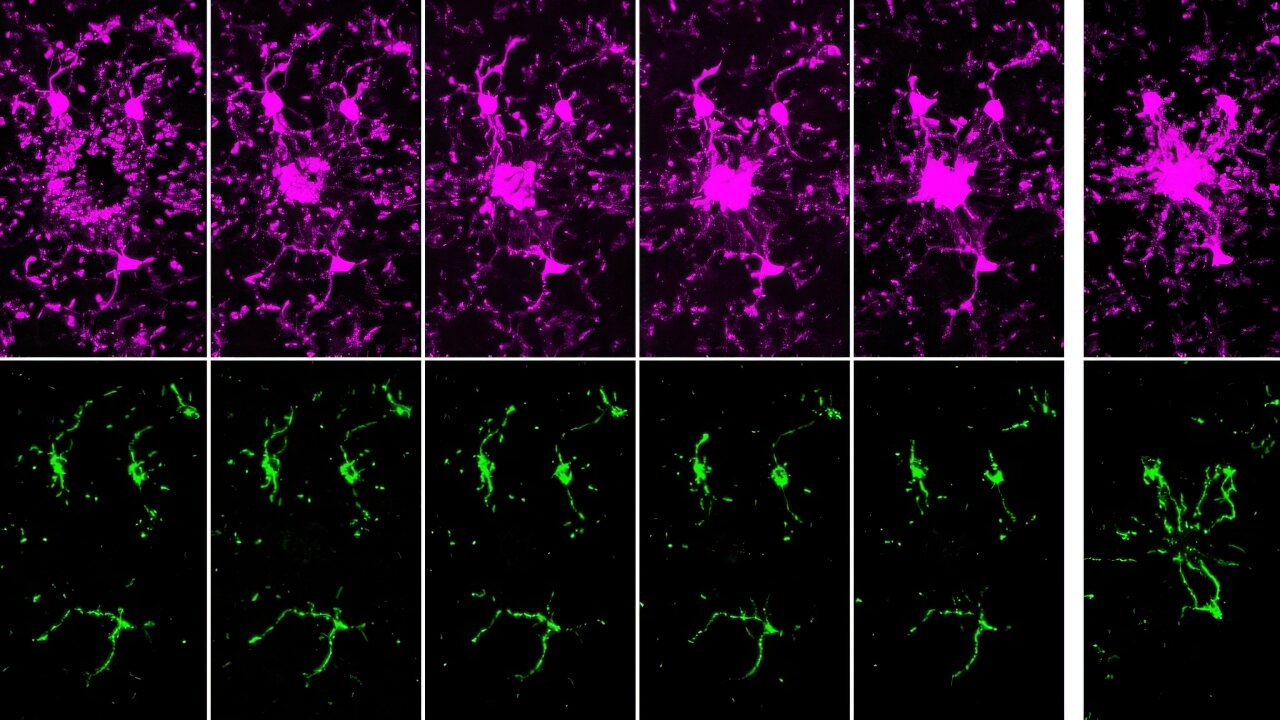
Macrophages, essential components of the innate immune system, rely heavily on energy generated by their mitochondria to engulf and eliminate pathogens. However, the presence of 2-AA significantly reduces adenosine triphosphate (ATP) levels in these cells, disrupting their bioenergetics.
ATP, the cell’s energy currency, is primarily produced through two pathways: glycolysis in the cytoplasm and oxidative phosphorylation in the mitochondria. While glycolysis yields a modest 2 ATP molecules per glucose, mitochondrial oxidative phosphorylation generates approximately 30 ATP molecules per glucose, making it the most efficient energy source.
Using Seahorse assay technology, the researchers measured oxygen consumption—a hallmark of oxidative phosphorylation—and observed a significant drop in cells exposed to 2-AA. This indicated that 2-AA specifically targets mitochondrial energy production, effectively shutting down the more efficient ATP-generating pathway.
Additionally, 2-AA was found to block pyruvate transport into mitochondria, leaving excess pyruvate in the cytoplasm. Pyruvate is a critical metabolite that fuels the Krebs cycle and oxidative phosphorylation within mitochondria. Without it, the energy output from macrophage mitochondria is severely impaired, leading to diminished immune responses.
Mitochondrial Dysfunction in Living Systems
To validate these findings, Rahme and her team conducted experiments in mice infected with either wild-type P. aeruginosa or a mutant strain unable to produce 2-AA. In mice infected with the wild-type bacteria, ATP levels in the spleen—a key organ for immune responses—dropped within 24 hours, while those infected with the mutant strain maintained normal energy levels.
Further analysis revealed reduced levels of acetyl-cofactor A, a mitochondrial metabolite derived from pyruvate, in wild-type infections. This confirmed that 2-AA disrupts mitochondrial bioenergetics. Importantly, the absence of 2-AA allowed macrophages to combat bacterial infections more effectively, leading to a lower bacterial burden by day 10.
Implications for Mitochondria-Targeted Therapies
As antibiotic resistance continues to rise, targeting host-pathogen interactions presents a promising therapeutic strategy. Kayeen Vadakkan, a microbiologist at St. Mary’s College, Thrissur, who was not involved in the study, highlighted the potential of targeting 2-AA to enhance mitochondrial function in macrophages. “We can complement our immune system,” Vadakkan said, suggesting that blocking 2-AA could restore macrophage energy production and improve their ability to fight infections.
Rahme’s laboratory is developing inhibitors of MvfR, a transcription factor necessary for 2-AA production. Early results show promise, but further studies are needed to evaluate their clinical safety and efficacy.
Beyond infection, 2-AA’s ability to suppress mitochondrial activity and reduce inflammation hints at its potential application in treating autoimmune diseases like rheumatoid arthritis and lupus, where overactive macrophages drive inflammation.12 “2-AA is a molecule which is anti-inflammatory in nature,” Chakraborty noted, emphasizing its dual therapeutic potential.