Exploring Age-Related Changes in Mitochondrial Structure and Function
Mitochondria, essential organelles with dual membranes, are the energy powerhouses of cells. Their inner membrane folds, known as cristae, are crucial for enhancing the organelle’s energy production capabilities.
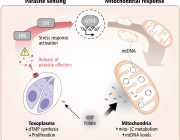
While oxidative stress is a natural byproduct of mitochondrial activity, excessive stress can lead to dysfunction, aging, and diseases. The structural and functional changes in mitochondria vary significantly across different tissues.

The study, published in Advanced Biology, revealed that while overall mitochondrial size increases with age, the surface area, volume, and complexity of cristae decrease, which affects thermogenic capacity and overall metabolic health.
Changes in Cristae and Mitochondrial Structure
The study discovered that while the overall size of mitochondria increases with age, the surface area, volume, and complexity of cristae diminish. This structural change impacts the mitochondria's efficiency and functionality in energy production.
Influence on Thermogenic Capacity
Mitochondrial shape was found to significantly affect the thermogenic capability of brown adipose tissue (BAT). Round or spherical mitochondria were associated with higher thermogenic function, whereas elongated mitochondria showed reduced thermogenic capacity and were more common in older subjects.
Future Perspectives
- Exploration of Sex-Dependent Differences: The research team plans to investigate whether there are differences in mitochondrial structure and function that are dependent on sex as individuals age.
- Broader Implications for Aging and Metabolic Health: By studying mitochondrial structure across different tissues and species, the team hopes to gain a deeper understanding of aging mechanisms and identify potential therapeutic targets for treating age-related ailments and mitochondrial dysfunctions.
Stay tuned for Targeting Mitochondria 2024 this October for more updates mitochondria reseacrh and aging.
HDL-C and Ferritin: Key Metabolic Indicators of Long COVID-19 Severity Revealed, Opening New Avenues for Treatment Strategies
Study reveals HDL-C and ferritin as crucial markers for long COVID-19 severity, leading to novel treatment strategies.

Long COVID-19, or post-acute sequelae of SARS-CoV-2 infection (PASC), is a global health phenomenon characterized by persistent symptoms following the acute phase of COVID-19. Affecting millions worldwide, it leads to sustained healthcare needs and impacts workforce participation due to symptoms such as fatigue and cognitive impairments. The condition highlights the necessity for ongoing research, healthcare system adjustments, and the formulation of targeted treatments to address its prolonged effects on individuals and economies.
A recently published study in Clinics Journal (Elsevier) has shed light on significant metabolic changes in non-vaccinated individuals with Long COVID-19, offering key insights into disease severity. This study was led by Marvin Edeas, MD, PhD, Université de Paris, Institut Cochin, INSERM 1016, France, and a team of international researchers led by Jumana Saleh, PhD, Sultan Qaboos University Hospital, Oman.
Published under the title “Reduced HDL-cholesterol in Long COVID-19: A Key Metabolic Risk Factor Tied to Disease Severity”, the study examined 88 patients across varying degrees of initial disease severity (mild, moderate, and severe) compared to a control group comprising 29 healthy individuals.
Findings from the controlled study revealed major metabolic shifts, particularly a substantial reduction in HDL-cholesterol (HDL-C) levels, coupled with a twofold increase in ferritin levels and insulin resistance among severe Long COVID-19 cases, compared to milder cases and the control group. These metabolic markers emerged as leading predictors of disease severity, offering novel understandings of Long COVID-19 management and treatment.
Marvin Edeas explained, “Our research has, for the first time, established a direct correlation between HDL-C and ferritin levels and the severity of Long COVID-19. The decline in HDL-C levels and the rise in ferritin levels observed in patients, influenced by the severity of the initial infection, could potentially play a role in the persistence and progression of Long COVID-19 symptoms”. This study is critical in understanding Long COVID-19 and its long-term impacts on metabolic health.
The research findings suggest that HDL-C and ferritin levels could serve as crucial markers and therapeutic targets, opening new avenues for treatment strategies aimed at mitigating the long-term effects of the disease. By considering these metabolic markers, we can shape preventive strategies and significantly mitigate the long-term impacts of COVID-19 on patients’ health.
The Function of HDL-C in Immune Response Modulation
The observed correlation between diminished levels of HDL-cholesterol (HDL-C), the severity of COVID-19, and its prolonged course might be explained by HDL-C's function as a modulator of the immune response. This includes its roles as an anti-inflammatory, antioxidant, and antiatherogenic agent, particularly vital during the heightened inflammatory response triggered by the virus. Investigating HDL-C’s utility beyond its conventional role in cholesterol transport is crucial for a comprehensive understanding of COVID-19 and its secondary health effects, such as long-COVID.
Implications of Lipid Remodeling During SARS-CoV-2 Infection
Extensive research indicates that COVID-19 precipitates notable shifts in the host's lipid metabolism, leading to the accumulation of cellular lipid reserves. These alterations aid in the viral takeover of host cellular mechanisms, thus facilitating the progression of the infection. This theory gains support from laboratory evidence showing the cessation of viral replication upon the administration of small molecule lipid inhibitors, highlighting the critical dependence of the virus on host lipid resources for replication.
The Interrelation of Iron Dysregulation, HDL-C, and Ferroptosis in COVID-19
A notable aspect of the interplay between HDL-C functionality and iron homeostasis is the process of ferroptosis, induced by lipid peroxidation and disturbed iron balance, characterized by the buildup of iron and products of lipid oxidation. This leads to diminished antioxidant defense capabilities. HDL-C is influential in mitigating the detrimental effects associated with ferroptosis, underscoring the significance of maintaining balanced iron levels in COVID-19 management.
"Our findings highlight the exacerbating effect of impaired iron regulation on COVID-19 progression, further complicated by the disrupted protective functions of HDL-C", stated Jumana Saleh.
The Dynamic Competition Between Host Metabolic Processes and Viral Interference
The outcome of the "war", between the host's metabolic defenses and viral invasion strategies, axes on the control over iron and lipid resources. The virus strategically targets these metabolic reserves to support its replication and spread. For Marvin Edeas, this battle underscores the complex interaction between host metabolic pathways and viral mechanisms, emphasizing the strategic importance of iron and lipid regulation in determining the course and outcome of COVID-19 infection.

Marvin Edeas, Founder of the WMS
How does the strategic alteration of iron and HDL-C levels by a virus contribute to its underlying aim of targeting mitochondria to disrupt host defense mechanisms?
Marvin Edeas commented on the perspective of this study.
"In the intricate dance of viral infection, the virus employs a calculated strategy aimed directly at the heart of the host's cellular energy and defense systems — the mitochondria. By subtly manipulating and altering the host's iron metabolism and HDL-C levels, the virus orchestrates a multifaceted attack designed to undermine mitochondrial integrity and function. This strategic disruption serves to weaken the mitochondria, a crucial step in the virus's broader aim to compromise the host's ability to mount an effective defense. Through this sophisticated mechanism of action, the virus ensures its survival and proliferation within the host, highlighting the importance of understanding these viral tactics for the development of targeted therapeutic interventions".
The implications of this study are broad, providing a new understanding of Long COVID-19’s impact on metabolic health and laying the foundation for future research and therapeutic interventions aimed at improving patient outcomes.
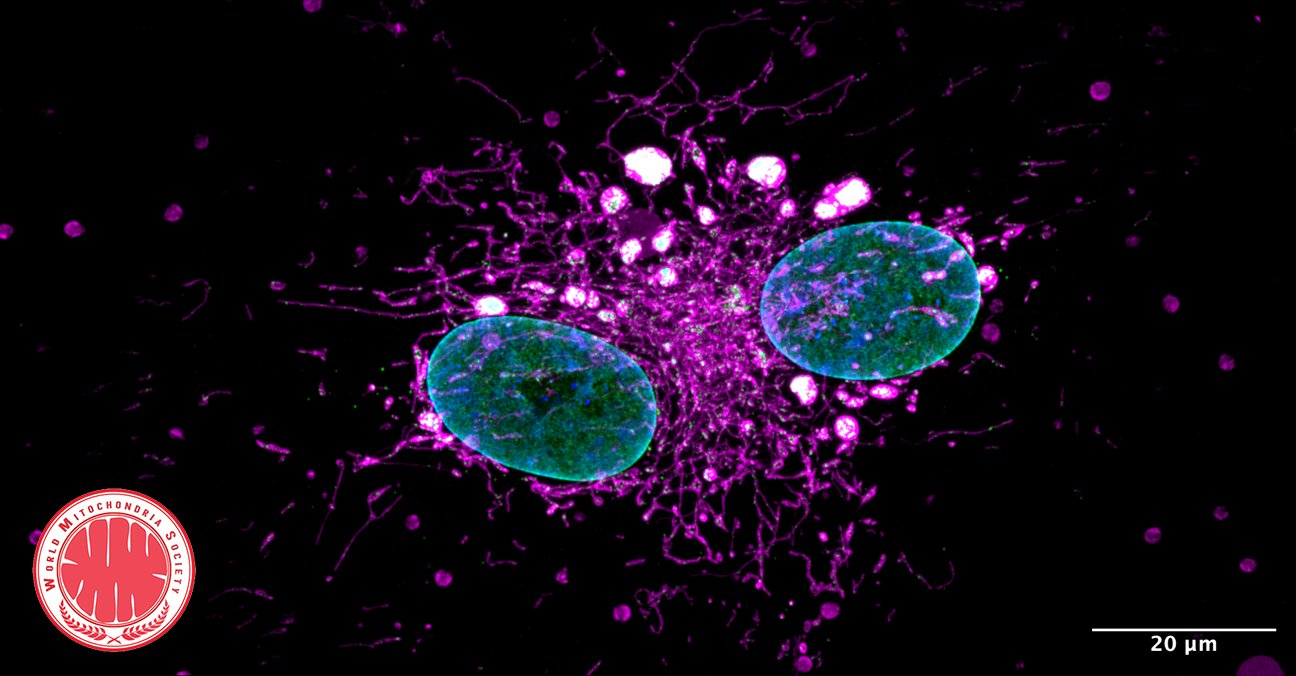
Mitochondria and Powering the Mind: Secrets of Synaptic Energy with VAP
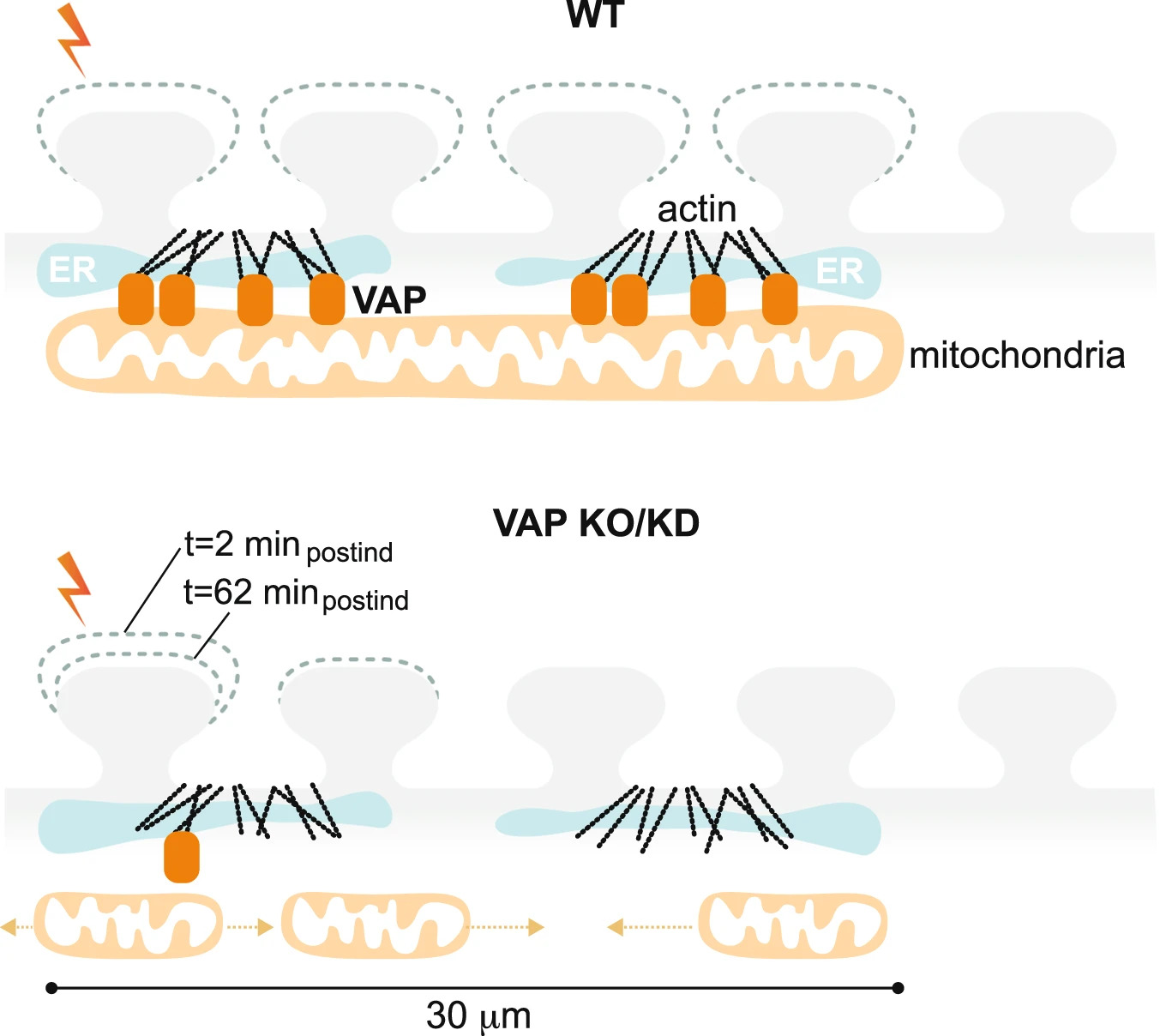
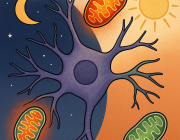
VAP spatially stabilizes mitochondria to locally support synaptic plasticity.
WMS Analysis
The study highlights the essential role of VAP (vesicle-associated membrane protein-associated protein) in synaptic plasticity by stabilizing mitochondria near synapses through cytoskeletal tethering. VAP's ability to maintain mitochondrial stability is crucial for meeting the high energy demands of synaptic activity, facilitating memory formation and learning. This stabilization not only supports the immediate demands of synaptic plasticity but also acts as a determinant for the spatial extent of dendritic segments involved in these processes. The implications of these findings extend to understanding the mechanisms underlying neurodegenerative diseases like ALS, where disruptions in mitochondrial stability at synapses could contribute to disease pathology. Essentially, the research underscores the critical interplay between mitochondrial stability, synaptic plasticity, and the potential for targeted therapies in neurodegenerative diseases.
About the study
The study investigates the crucial role of synapses in plasticity and memory formation, highlighting that synapses, being energy consumption hotspots, depend on local energy supplies provided by mitochondria. These mitochondria are stabilized near synapses by the cytoskeleton, a necessary arrangement for supporting synaptic plasticity. The research identifies proteins that exclusively tether mitochondria to the actin near postsynaptic spines, with a focus on VAP (vesicle-associated membrane protein-associated protein), known for its implications in amyotrophic lateral sclerosis (ALS). VAP is shown to stabilize mitochondria via actin near the spines, playing a vital role in maintaining mitochondrial compartments that locally support synaptic plasticity.
The study elaborates on the necessity of local energy sources for synapses, given their distance from the neuron's cell body and high energy demands. It was previously established that mitochondria form temporally and spatially stable compartments by tethering to the cytoskeleton, which are crucial for fueling synaptic plasticity. However, the mechanisms enabling these stable mitochondrial compartments in dendrites were not fully understood.
This research provides significant insights by identifying the role of VAP in stabilizing dendritic mitochondria, ensuring their function during long durations of synaptic plasticity formation and maintenance. VAP's unique role includes acting as a spatial stabilizer and a ruler, determining the extent of dendritic segments supported during synaptic plasticity, crucial for clustered synaptic plasticity related to learning and development.
Furthermore, the study underscores the broader implications of mitochondrial dysfunction in neurodegenerative diseases like Alzheimer's, Parkinson's Disease (PD), and ALS. By leveraging advances in proteomic labeling and high-resolution imaging techniques, the research delineates the mechanisms dictating the spatial organization of dendritic mitochondria and their pivotal role in sustaining synaptic plasticity. The findings emphasize VAP's distinct role in spatially stabilizing mitochondria in dendrites, influencing both the formation and maintenance of synaptic plasticity and potentially offering new avenues for understanding and treating neurodegenerative diseases.
Photo Credits: Bapat, O., Purimetla, T., Kruessel, S. et al. Nat Commun15, 205 (2024).
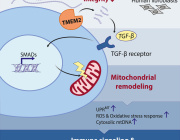
Transforming Transplantation: Real-Time Monitoring of Mitochondrial Health During Organ Perfusion
A recent study published in Nature Scientific Reports journal addressed a significant challenge in the field of organ transplantation – ischemia reperfusion injury (IRI) – which can lead to post-transplantation complications and limit the use of organs from extended criteria donors.
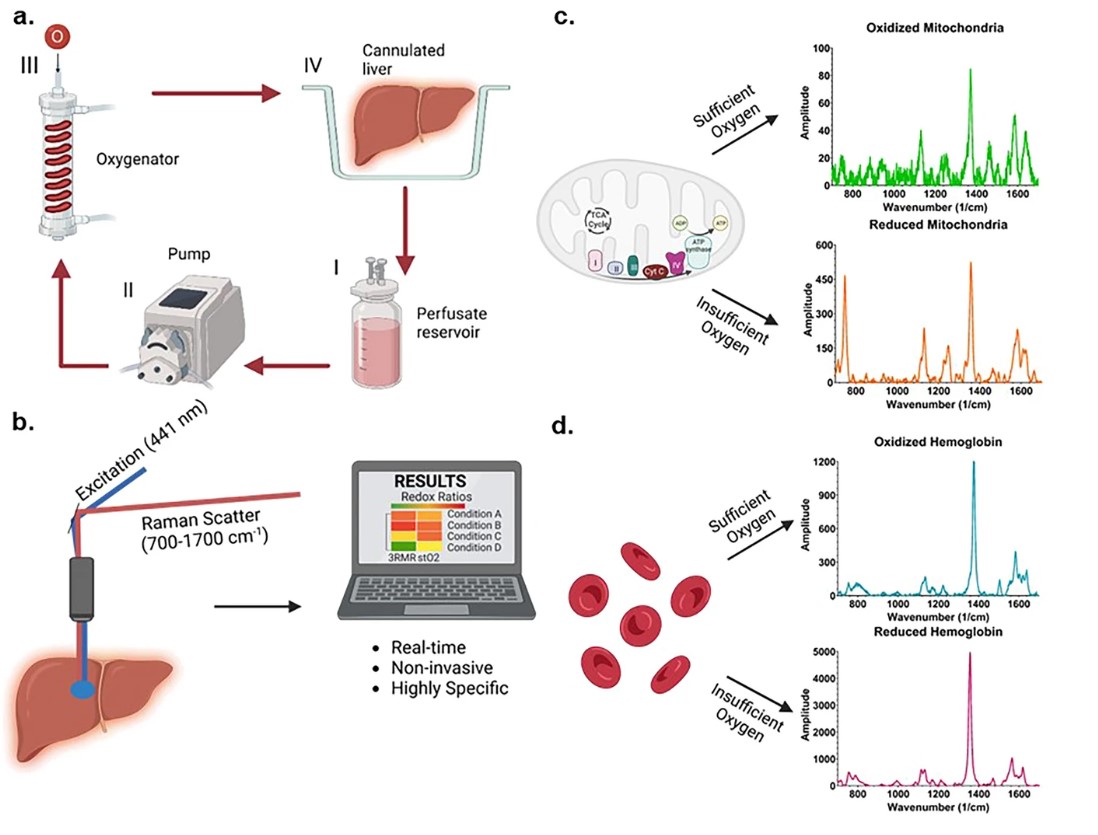
Resonance Raman Spectroscopy predicts functional oxygenation of liver tissue during machine perfusion. Credits: Rohil Jain et al., 2024
A team of researchers from Harvard Medical School, Massachusetts General Hospital and Shriners Children’s Hospital in the USA has developed a novel, real-time, and non-invasive method to assess organ quality during machine perfusion. This innovative approach focuses on mitochondrial function and injury, using resonance Raman spectroscopy to quantify the oxidation state of mitochondrial cytochromes during perfusion.
The new technique, known as the index of mitochondrial oxidation or 3RMR, was employed to study the differences in mitochondrial recovery of cold ischemic rodent livers during machine perfusion at normothermic temperatures. The study compared acellular and cellular-based perfusates to understand their impact on organ quality.
The findings revealed that, following 24 hours of static cold storage, the 3RMR returned to baseline faster with a cellular-based perfusate. However, the 3RMR progressively increased during perfusion, indicating that injury may develop over time. These insights underscore the need for further refinement of reoxygenation strategies during normothermic machine perfusion, taking into account cold ischemia durations, gradual recovery/rewarming, and the risk of hemolysis.
These findings highlight an improved organ assessment during machine perfusion, ultimately enhancing transplantation outcomes and expanding the pool of viable organs for those in need.
Stay tuned for Targeting Mitochondria 2024 this October for more updates mitochondria and the future of organ transplantation.
Mitochondrial Dynamics Crucial in Determining Muscle Fiber Type, Study Finds
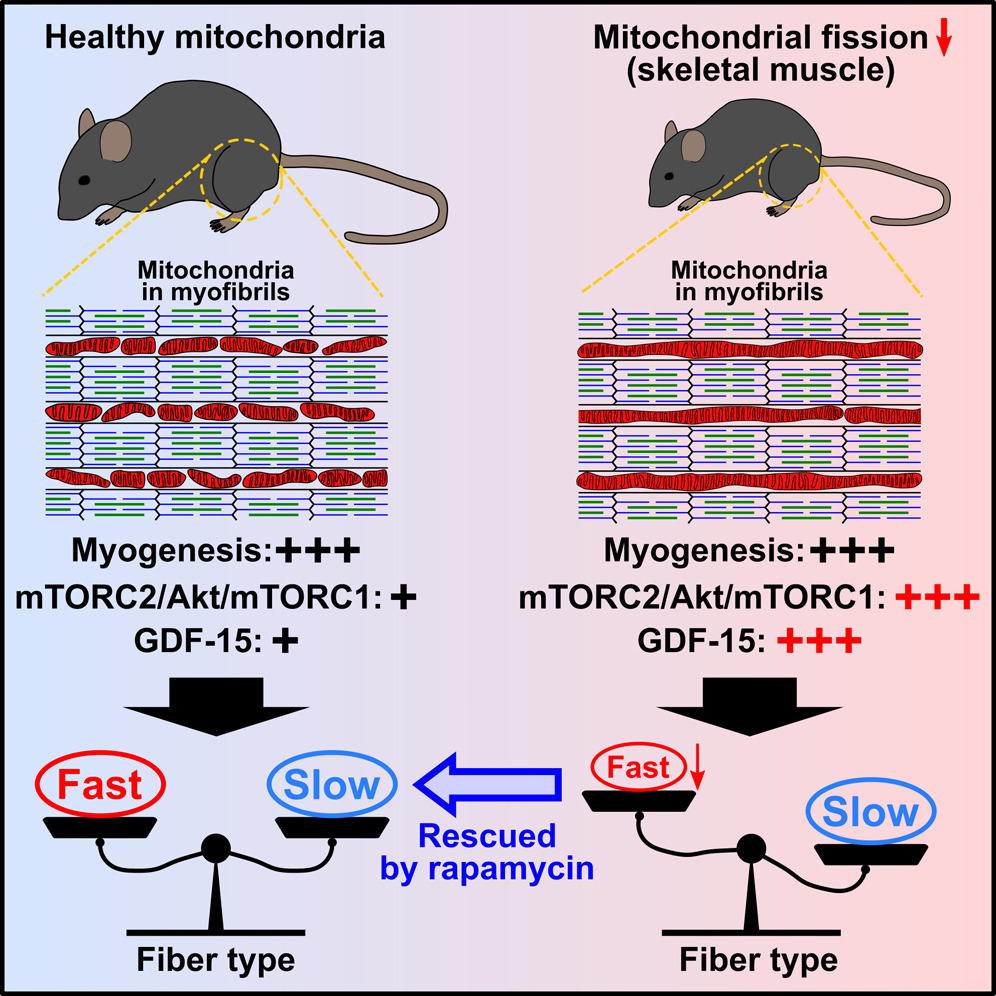
In a recent study published in Cell Reports, researchers led by Naotada Ishihara from Osaka University, Japan, shed light on the intricate relationship between mitochondrial dynamics and muscle fiber type differentiation.
The study uncovered that the loss of mitochondrial fission, a process controlled by dynamin-related protein 1 (Drp1), specifically hampers the differentiation of fast-twitch muscle fibers. This finding challenges previous notions, emphasizing the pivotal role of mitochondrial dynamics in shaping muscle composition post-birth.
By depleting Drp1 in both mouse skeletal muscle and cultured myotubes, the researchers observed a distinct reduction in fast-twitch fibers, independent of respiratory function. This shift in fiber type was accompanied by the activation of the Akt/mammalian target of rapamycin (mTOR) pathway, facilitated by the accumulation of mTOR complex 2 (mTORC2) on elongated and bulb-like mitochondria.
Excitingly, intervention through rapamycin administration effectively rescued the decline in fast-twitch fibers both in vivo and in vitro, highlighting the potential for targeted therapies in muscle-related disorders.
Furthermore, the study identified the upregulation of growth differentiation factor 15 (GDF-15), a mitochondria-related cytokine, under Akt/mTOR activation. This upregulation, in turn, suppressed the differentiation of fast-twitch fibers, unraveling a previously unknown regulatory mechanism in muscle development.
Overall, these findings elucidate the critical role of mitochondrial dynamics in activating mTORC2 on mitochondria, ultimately dictating the differentiation of muscle fibers. The study not only enhances our understanding of muscle biology but also unveils potential avenues for therapeutic interventions in muscle-related pathologies.
More Articles...
- The Secrets of Aging: Mitochondrial DNA Release and Cellular Senescence
- Revolutionizing Cancer Treatment: Enhancing Immune Response through Mitochondrial Manipulation
- Non-Invasive Photobiomodulation Therapies for Diabetes Management and Complication Reduction
- Intranasal Delivery of Mitochondria-Targeted Neuroprotective Drugs: A New Approach for Treating Traumatic Brain Injury