The Secrets of Aging: Mitochondrial DNA Release and Cellular Senescence
A recent National Institute of Aging funded study, published in Nature on February 29, 2024, reveals that mitochondrial DNA (mtDNA) release is a key factor in cellular senescence and inflammation in mice. This process contributes to the aging-related dysfunction and disorders by activating changes in senescent cells. When this leakage of mtDNA is inhibited in aged mice, there's a noticeable reduction in inflammation, an improvement in bone health, and a decrease in overall frailty. The study highlights apoptosis and the process of widespread mitochondrial outer membrane permeabilization (MOMP) as essential in this context. It demonstrates how mtDNA released during MOMP triggers changes leading to cell death and immune clearance.
The research, led by the Mayo Clinic, identifies the escape of aging cells from apoptosis, which leads to their accumulation and harmful effects on neighboring cells and tissues through the senescence-associated secretory phenotype (SASP). By examining the role of mitochondria in senescence and SASP, it was discovered that inhibiting mtDNA release could suppress these aging effects. Treating old mice with compounds that prevent mitochondrial pore formation resulted in reduced brain inflammation and improved musculoskeletal health.
These findings offer new insights into mitochondrial function in cellular aging and suggest potential therapeutic strategies for mitigating age-related conditions. Further investigation is required to understand these mechanisms in humans and explore the therapeutic potential of inhibiting mtDNA release in senescent cells.
This hot topic will be a subject of discussion at the upcoming World Mitochondrial Society (WMS) meeting in Berlin, where experts will discuss the implications of mitochondrial DNA release on aging and potential interventions.
Image Credits: NIH, National Institute on Aging.
World Mitochondria Society
Annual World Congress on Targeting Mitochondria
LinkedIn | Facebook
Revolutionizing Cancer Treatment: Enhancing Immune Response through Mitochondrial Manipulation

In a study published in Science, researchers led by Kailash Chandra Mangalhara and Gerald S. Shadel explored a groundbreaking approach to boosting the body's immune response against cancer by tweaking how mitochondria in cancer cells handle energy. They focused on a part of the cell's power plant called the mitochondrial electron transport chain (ETC), which is crucial for powering immune cells to fight off invaders. By adjusting the flow of electrons within the mitochondria of cancer cells, specifically through a segment known as complex I, the team found a way to increase the levels of a molecule called succinate. This, in turn, activated important immune system genes in the cancer cells, making them more visible and vulnerable to attack by the body's T cells.
This discovery is significant because it sidesteps the need for interferon-gamma, a common but sometimes problematic component in cancer immunotherapy. Instead, the method relies on a more direct modulation of cancer cell properties to stimulate an immune attack. Specifically, by altering a regulatory protein within the mitochondria, the researchers could enhance the immune system's ability to detect and destroy melanoma cells without adversely affecting noncancerous cells. This strategy opens new avenues for treating cancers that have become adept at evading the immune system, potentially offering a more targeted and less side-effect-prone therapy option.
© News Copyright: World Mitochondria Society (WMS)
Intranasal Delivery of Mitochondria-Targeted Neuroprotective Drugs: A New Approach for Treating Traumatic Brain Injury
Researchers led by Jignesh D. Pandya, have identified a promising method for delivering neuroprotective drugs directly to the brain for treating traumatic brain injury (TBI) through intranasal administration, bypassing the blood-brain barrier (BBB).
This non-invasive approach enhances drug bioavailability, reduces the need for high doses, and minimizes adverse side effects.
Focusing on compounds targeted at mitochondria—key regulators of cell death and damage following TBI—the study reviews the advantages of intranasal delivery over traditional methods.
It highlights successful applications in animal models, such as reducing stroke damage and reversing Alzheimer’s symptoms, and outlines the screening of compounds based on pharmacological properties for intranasal use.
This innovative strategy could significantly impact the treatment of TBI, especially in military settings, by offering a rapid, effective, and field-applicable method to counteract the progression of TBI pathogenesis.
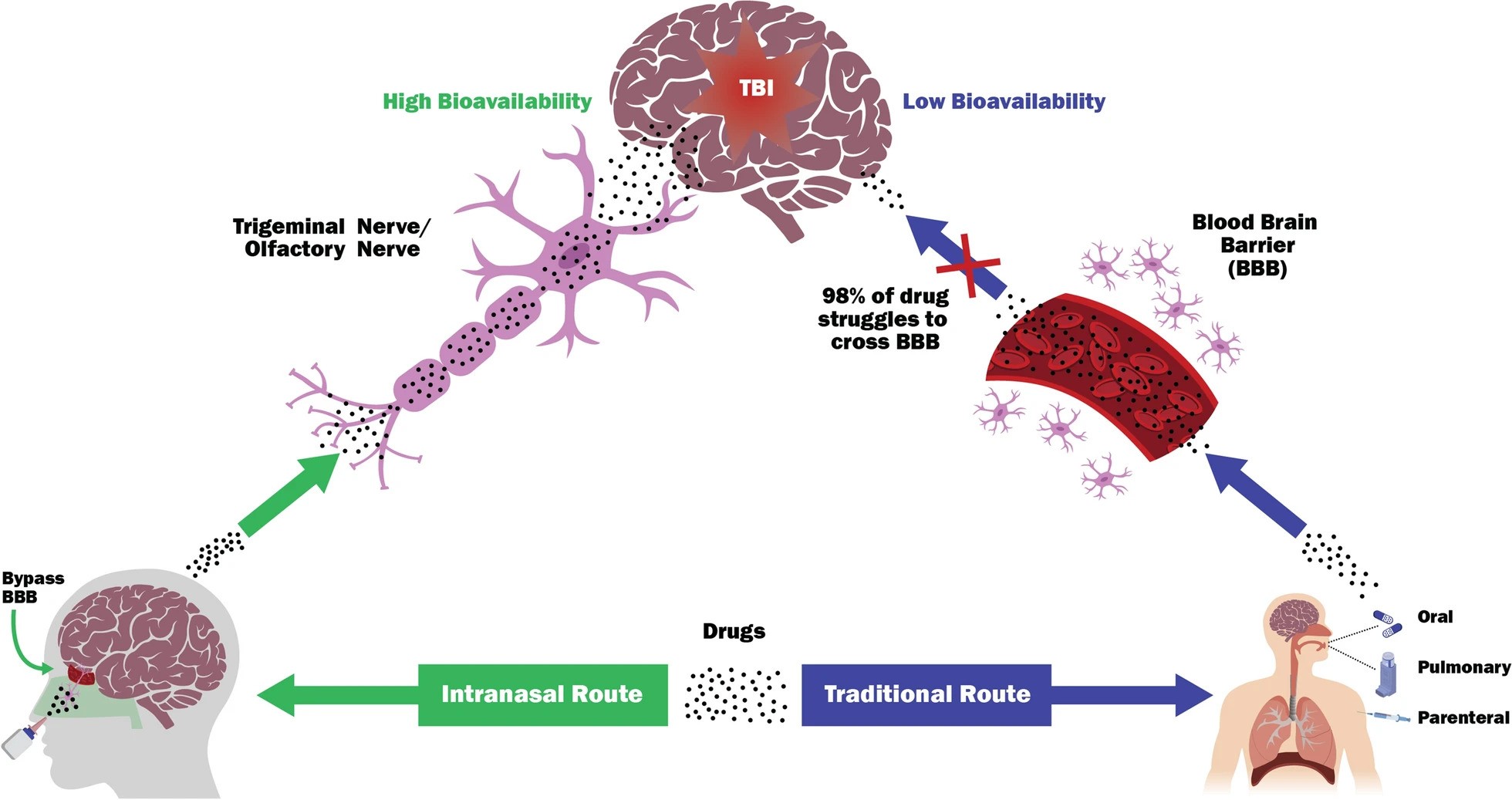
Image Description:
Schematic representation of key aspects of intranasal delivery of neuroprotection compounds to the brain. TBI is difficult to treat as most therapeutic agents (98%) cannot reach in the brain, mainly due to the selective permeability of the blood–brain barrier (BBB). The olfactory and trigeminal nerves can serve as direct nose-to-brain routes that bypass the BBB that can impede absorption of most CNS targeted compounds, resulting in higher bioavailability. In addition, compared to traditional routes, the nasal administration of drugs can direct the rapid CNS absorption to brain tissues, thereby circumventing the hepatic first-pass metabolism and gastric degradation and allowing fast onset of pharmacological action.
Image Credits: Pandya, J.D., Musyaju, S., Modi, H.R. et al. J Transl Med22, 167 (2024).
© World Mitochondria Society (WMS)
Non-Invasive Photobiomodulation Therapies for Diabetes Management and Complication Reduction
The WMS wish to highlight 2 excellent studies just published by 2 different teams.
Recent studies have highlighted the potential of photobiomodulation (PBM) and transcranial photobiomodulation (tPBM) as non-invasive therapeutic interventions for diabetes management and the mitigation of its complications. These innovative approaches focus on improving mitochondrial function, insulin therapy outcomes, and systemic metabolic health through light-based stimulation.
Key Findings
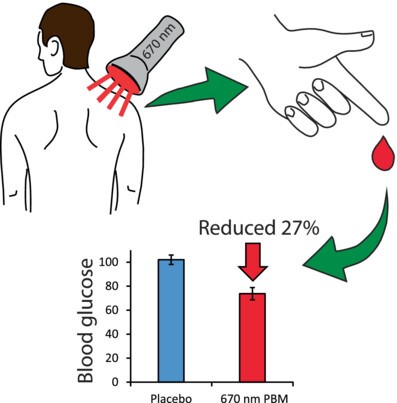
1. Blood Glucose Management via PBM
Research by Michael B. Powner and Glen Jeffery demonstrates that PBM with 670 nm light significantly reduces blood glucose levels following glucose intake, by enhancing mitochondrial functions and potentially increasing glucose demand. This method showed a notable decrease in blood glucose spikes, offering a new avenue for managing post-meal blood glucose fluctuations.
2. tPBM's Role in Microglial Function and Diabetic Complications
A study by Shaojun Liu, Dongyu Li, et al., explored tPBM's effectiveness in improving microglial morphology and reactivity in diabetic mice. The treatment was found to stimulate the brain's drainage system, enhance energy expenditure, and improve locomotor activity, suggesting tPBM as an effective method for treating microglial dysfunction and potentially preventing diabetic physiological disorders.
3. Broad Therapeutic Effects of tPBM
Additional research supports tPBM's beneficial effects on various diabetic complications, including diabetic foot, periodontitis, and retinopathy. tPBM has also been shown to improve insulin sensitivity and metabolic disorders in adipocytes and high-fat diet-induced mice. The underlying mechanisms involve the activation of cytochrome c oxidase-mediated protein kinase B, leading to increased mitochondrial ATP and ROS generation, lipid consumption, glucose absorption, and glycogen accumulation.
Conclusion
The integration of PBM and tPBM into diabetes management strategies offers a promising outlook for both controlling blood glucose levels and addressing a spectrum of diabetic complications. These non-invasive therapies not only provide a novel approach to enhancing metabolic health but also contribute to the broader effort of improving quality of life for individuals with diabetes. The evidence suggests that further exploration and clinical trials could solidify the role of photobiomodulation therapies in diabetes care protocols, potentially revolutionizing treatment methodologies with their systemic benefits and minimal side effects.
The mitochondria continue to astonish us.
References
1. Powner, M.B., & Jeffery, G. (2024). Light stimulation of mitochondria reduces blood glucose levels. Journal of Biophotonics, 10.1002/jbio.202300521.
2. Liu, S., Li, D., ... Zhu, D. (2023). Transcranial photobiomodulation improves insulin therapy in diabetic microglial reactivity and the brain drainage system. Communications Biology, 6, Article number: 1239.
Photo Credits:
Powner, M.B., & Jeffery, G. (2024).
© News Copyright: World Mitochondria Society (WMS)
How Obesity Dismantles Our Mitochondria
These colored streaks are mitochondrial networks within fat cells. Researchers from UC San Diego discovered that a high-fat diet dismantles mitochondria, resulting in weight gain. Photo credit: UC San Diego Health Sciences
The number of people with obesity has nearly tripled since 1975, resulting in a worldwide epidemic. While lifestyle factors like diet and exercise play a role in the development and progression of obesity, scientists have come to understand that obesity is also associated with intrinsic metabolic abnormalities. Now, researchers from University of California San Diego School of Medicine have shed new light on how obesity affects our mitochondria, the all-important energy-producing structures of our cells.
In a study published January 29, 2023 in Nature Metabolism, the researchers found that when mice were fed a high-fat diet, mitochondria within their fat cells broke apart into smaller mitochondria with reduced capacity for burning fat. Further, they discovered that this process is controlled by a single gene. By deleting this gene from the mice, they were able to protect them from excess weight gain, even when they ate the same high-fat diet as other mice.
“Caloric overload from overeating can lead to weight gain and also triggers a metabolic cascade that reduces energy burning, making obesity even worse,” said Alan Saltiel, PhD, professor in the Department of Medicine at UC San Diego School of Medicine. “The gene we identified is a critical part of that transition from healthy weight to obesity.”
Obesity, which affects more than 40% of adults in the United States, occurs when the body accumulates too much fat, which is primarily stored in adipose tissue. Adipose tissue normally provides important mechanical benefits by cushioning vital organs and providing insulation. It also has important metabolic functions, such as releasing hormones and other cellular signaling molecules that instruct other tissues to burn or store energy.
In the case of caloric imbalances like obesity, the ability of fat cells to burn energy starts to fail, which is one reason why it can be difficult for people with obesity to lose weight. How these metabolic abnormalities start is among the biggest mysteries surrounding obesity.
To answer this question, the researchers fed mice a high-fat diet and measured the impact of this diet on their fat cells’ mitochondria, structures within cells that help burn fat. They discovered an unusual phenomenon. After consuming a high-fat diet, mitochondria in parts of the mice’s adipose tissue underwent fragmentation, splitting into many smaller, ineffective mitochondria that burned less fat.
In addition to discovering this metabolic effect, they also discovered that it is driven by the activity of single molecule, called RaIA. RaIA has many functions, including helping break down mitochondria when they malfunction. The new research suggests that when this molecule is overactive, it interferes with the normal functioning of mitochondria, triggering the metabolic issues associated with obesity.
“In essence, chronic activation of RaIA appears to play a critical role in suppressing energy expenditure in obese adipose tissue,” said Saltiel. “By understanding this mechanism, we’re one step closer to developing targeted therapies that could address weight gain and associated metabolic dysfunctions by increasing fat burning."
By deleting the gene associated with RaIA, the researchers were able to protect the mice against diet-induced weight gain. Delving deeper into the biochemistry at play, the researchers found that some of the proteins affected by RaIA in mice are analogous to human proteins that are associated with obesity and insulin resistance, suggesting that similar mechanisms may be driving human obesity.
“The direct comparison between the fundamental biology we’ve discovered and real clinical outcomes underscores the relevance of the findings to humans and suggests we may be able to help treat or prevent obesity by targeting the RaIA pathway with new therapies,” said Saltiel “We’re only just beginning to understand the complex metabolism of this disease, but the future possibilities are exciting.”
News Source: University of California - San Diego.
World Mitochondria Society
Annual World Congress on Targeting Mitochondria
LinkedIn | Facebook
More Articles...
- Unlocking the Role of Mitochondria in Processing Dietary Fats
- HKDC1 Ensures Mitochondrial and Lysosomal Balance, Safeguarding Against Cellular Senescence
- Inflammation-Triggered Mitochondrial and Metabolic Disruptions Steering the Transition from Acute to Chronic Pain
- Zen and the art of mitochondrial maintenance: The machinery of death makes a healthier life




























































