Biomolecular Condensates Found to Safeguard Mitochondria During Aging
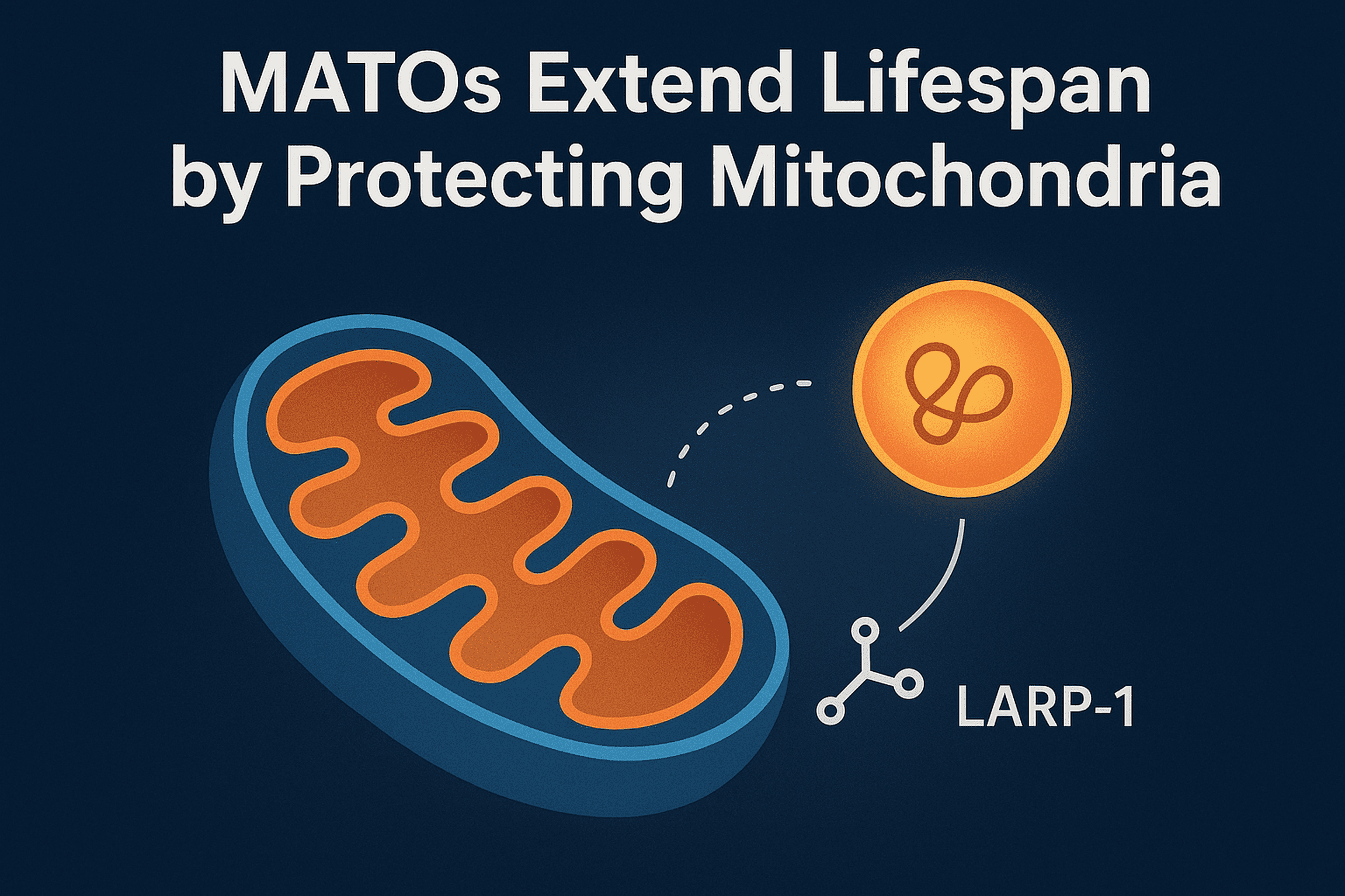
A new study in Nature Aging led by Prof. Chonglin Yang and colleagues has revealed a previously unknown mechanism that helps cells protect their powerhouses—mitochondria—during stress and aging.
The team discovered Mitochondria-Associated Translation Organelles (MATOs), a novel class of membraneless condensates formed by liquid–liquid phase separation (LLPS). These structures assemble directly on the mitochondrial surface, where they act as local protein factories.
At the center of this process is the RNA-binding protein LARP-1, which brings together ribosomes, mRNAs, and other RNA-binding proteins to produce crucial components for mitochondrial energy production and structure, such as IMMT-1 (a MICOS complex subunit for cristae organization) and ATP-2 (a key subunit of ATP synthase).
Using C. elegans as a model, the researchers showed that:
- Loss of LARP-1 or MATOs disrupts cristae organization, reduces ATP output, and shortens lifespan.
- By contrast, keeping MATOs anchored to mitochondria during stress or aging preserves mitochondrial function and significantly extends lifespan.
Mitochondrial decline is a hallmark of aging and contributes to neurodegeneration and metabolic disease. This discovery shows that biomolecular condensates directly regulate mitochondrial health, suggesting that stabilizing MATOs could become a new therapeutic strategy to combat age-related dysfunction.
Nature’s Boarding Pass: How Proteins Enter Mitochondria

Scientists Reveal Exciting New Rules for Mitochondrial Protein Delivery.
Caltech researchers have discovered a whole new layer in how proteins get to mitochondria, the energy centers of our cells. This finding challenges long-held beliefs and offers a glimpse into nature’s clever engineering.
For decades, scientists believed that mitochondrial proteins were made completely before being delivered. But the new study, published in Cell, shows that nearly 20 percent of these proteins begin their journey while still being made by ribosomes. This early delivery is called cotranslational import.
What makes this pathway special? The proteins that use it are usually large, complex, multi‑domain molecules, the kind that are difficult to fold correctly if fully formed out in the cell fluid. Folding them early and sending them off right away helps prevent them from getting stuck or forming tangled knots that could clog the mitochondrial gates.
But how does the cell know which proteins need this special route? It turns out there’s a two step signal system at play:
- A mitochondrial targeting sequence (think of it like a boarding pass).
- The appearance of a large folded domain the ‘code’ that unlocks the suitcase so the boarding pass can be used.
As Zikun Zhu, the lead author, puts it: “It’s like having your boarding pass locked in a suitcase. The targeting sequence is the boarding pass, but to access it you need the code to open the suitcase. In this case, the large domain is that code.”
In fact, when the researchers added one of these large domains to proteins usually imported later, they redirected them to the cotranslational pathway showing just how powerful this signal really is.
Unlike cotranslational targeting in other cell parts, mitochondrial targeting happens later only after the big domain is ready. This strategy ensures only the right proteins enter at the right time.
Looking ahead, the team plans to dive deeper into how this system works at a molecular level with hopes of one day tuning it for medical or biotechnological applications.
The topic will be further discussed at the World Mitochondria Society (WMS) annual meeting in Berlin, October 2025, where leading experts will explore how such discoveries are shaping the future of mitochondrial medicine.
Mitochondria May Be the Brain’s Secret Sleep Switch
By World Mitochondria Society
Why do we feel so tired when we don’t sleep?
A new study published in Nature suggests the answer may lie deep inside our brain cells, specifically in their mitochondria, the tiny energy-producing structures often called the cell’s “powerhouses.”
Researchers from the University of Oxford found that when fruit flies were sleep-deprived, a specific group of brain cells showed dramatic changes in their mitochondria. These neurons, which help control the urge to sleep, responded to sleep loss by ramping up energy production and reorganizing their internal machinery.
Mitochondria and Sleep Pressure
They discovered that sleep pressure, what makes us feel sleepy, builds up when mitochondria in key neurons become overworked.
After rest, these changes reversed, suggesting that mitochondria help the brain recover. Even more striking, the scientists were able to dial sleepiness up or down by changing how the mitochondria worked.
When the mitochondria were forced to split into smaller fragments, the flies slept less. When mitochondria were fused into longer structures, the flies felt a stronger need to sleep.
This discovery adds a surprising new piece to the puzzle of sleep science. Until now, sleep was mostly studied as a brain-wide process involving electrical signals or chemical messengers. But this study shows that the buildup of sleep pressure might begin with stress inside individual brain cells.
What’s next?
This research could one day help scientists design treatments for sleep disorders by targeting mitochondrial health. It also opens the door to rethinking sleep not just as a brain state, but as a metabolic response to cellular energy needs.
A new study published in Nature by Gero Miesenböch : https://www.nature.com/articles/s41586-025-09261-y
Mitochondria: Not Just the Powerhouse, but a Pathogen Defense Hub
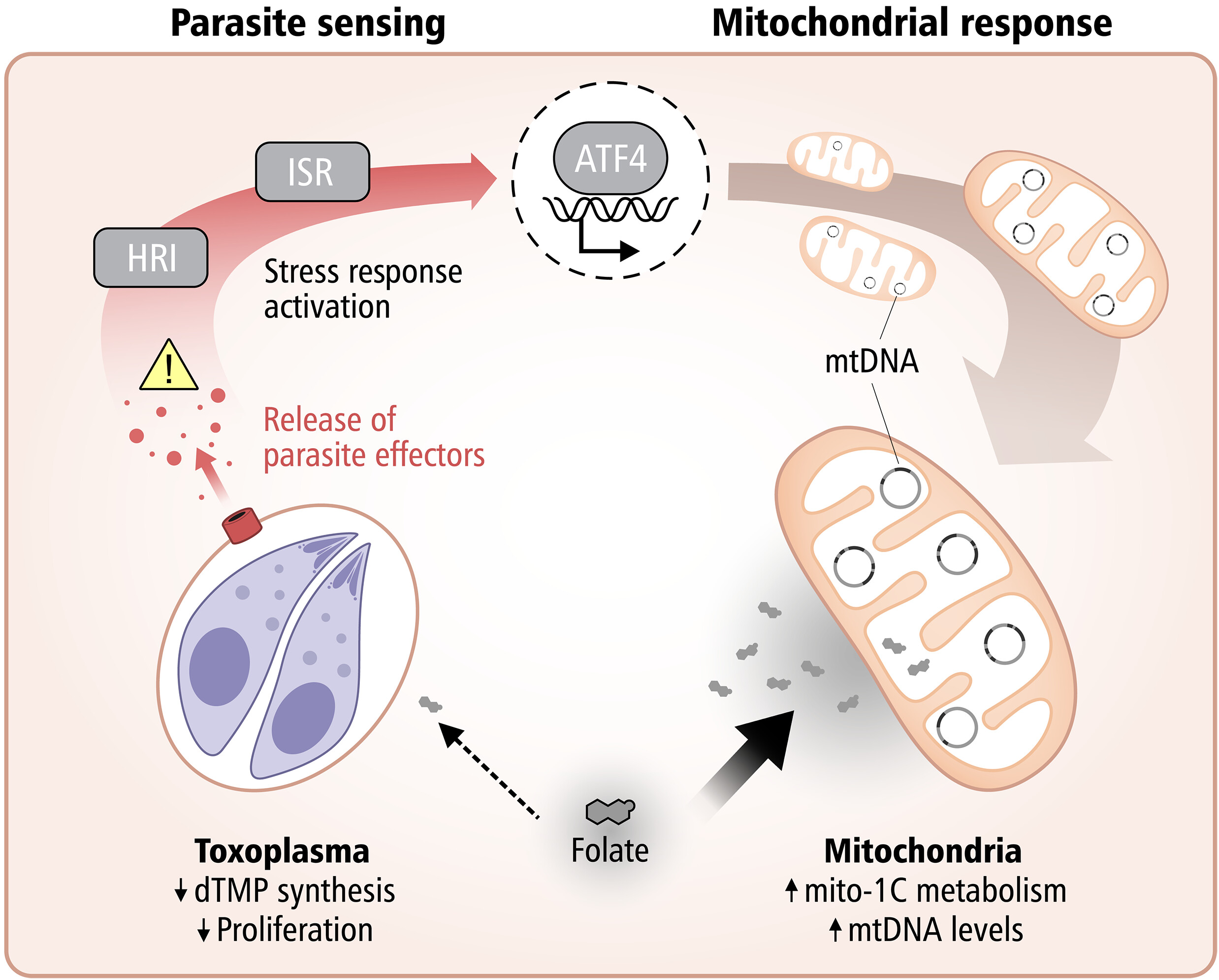
A fascinating new study published in Science from Lena Pernas’s group at the University of California Los Angeles reveals how our cells can starve intracellular parasites, by harnessing their own mitochondria to compete for essential nutrients.
The research demonstrates that mitochondria take on an active defensive role against the parasite Toxoplasma gondii by competing for folate, a vitamin critical for the parasite’s DNA synthesis.
When host cells detect infection, they trigger the integrated stress response, activating ATF4, which boosts mitochondrial one-carbon metabolism. This metabolic shift depletes host folate, effectively starving the parasite and limiting its replication.
This work highlights a striking example of metabolic immunity, where cells weaponize their own metabolic machinery to fight off infection.
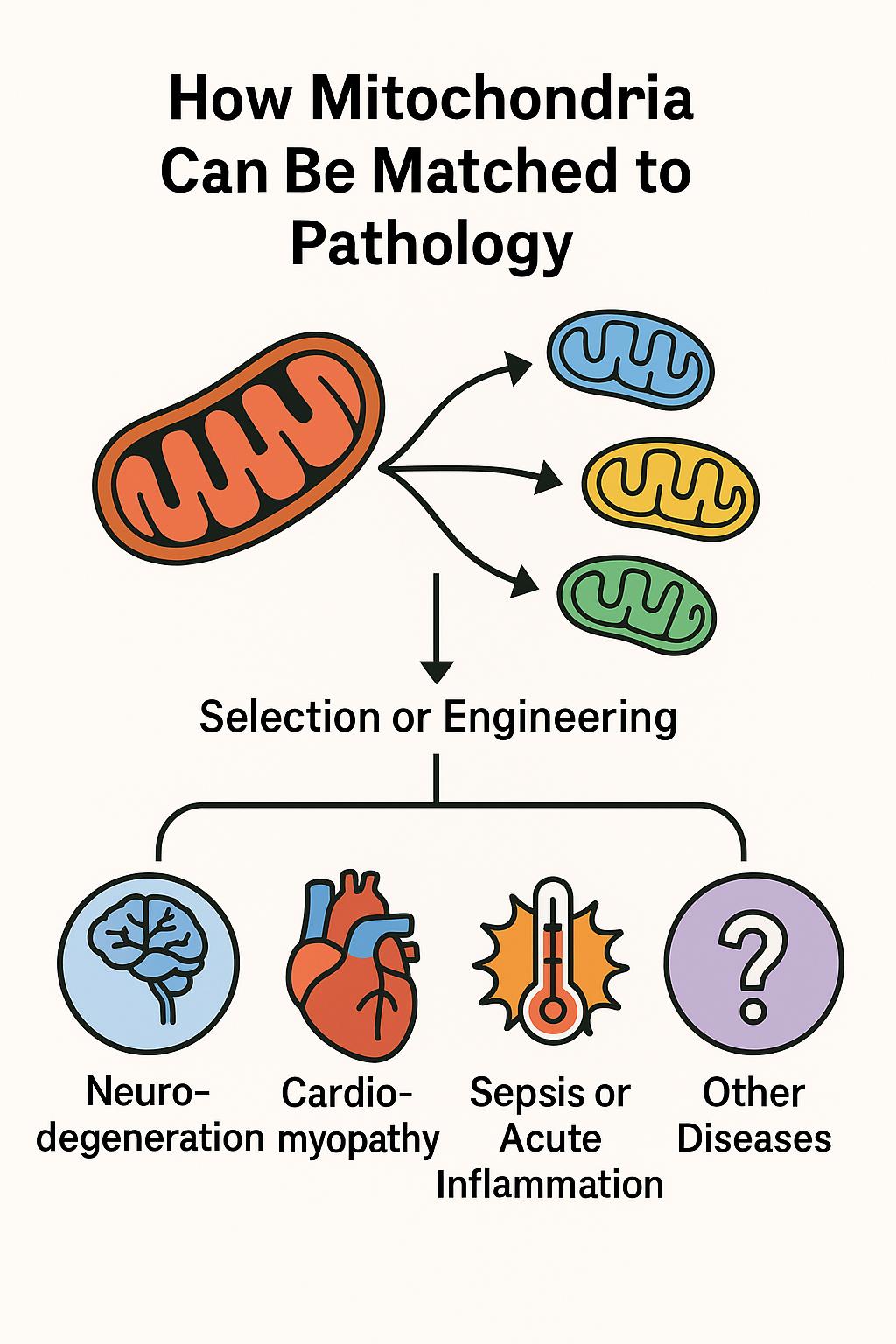
How Mitochondria Can Be Matched to Pathology
From Powerhouse to Precision Tool in Regenerative Medicine
Mitochondrial transplantation is rapidly emerging as a groundbreaking strategy in precision medicine. A recent study published in Cell Death & Disease demonstrates that mitochondria sourced from different cell types and species can exert distinct therapeutic effects, depending on the nature of cellular stress or damage—supporting the concept of pathology-matched mitochondrial therapy.
Led by Xiaomeng Lu the study demonstrates that mitochondria sourced from diverse species (canine, porcine, bovine, etc.) can be safely transplanted into stressed immune and cardiac cells, and even into live animal models, without triggering an immune response. More importantly, these transplanted mitochondria conferred distinct, context-dependent protective effects based on their origin—suggesting a future of tailored mitochondrial therapies.
This approach, referred to as adaptive bioenhancement, proposes that donor mitochondria can be selected or engineered to match the metabolic or immunological needs of specific diseases. The implications are profound: mitochondria are no longer seen merely as cellular batteries, but as programmable organelles capable of restoring balance in a targeted fashion.
Key Concepts Illustrated
• Cross-species delivery is safe and effective: The study found that mitochondria from various mammalian cells were well tolerated in both cell cultures and live mice—even across species barriers.
• Disease-specific matching is possible:
• Neurodegeneration: Mitochondria rich in antioxidant and synaptic-support functions.
• Cardiomyopathy: High-energy, calcium-buffering mitochondria.
• Sepsis & inflammation: ROS-scavenging, immunomodulatory mitochondria.
• Designer mitochondria: Fusion of mitochondrial populations (e.g. HL1 + H9C2) enhanced key proteins (Opa1, Drp1, Mfn1), enabling mitochondrial performance to be optimized for specific stress contexts.
In Vivo Confirmation
In a mouse model of LPS-induced acute inflammation, tail-vein injection of wild-derived mitochondria:
• Boosted grip strength and endurance
• Reduced IL‑6, TNF‑α, and IL‑10 serum levels
• Normalized liver and kidney function markers
• Reduced tissue ROS and lipid peroxidation
Global Perspective – Berlin 2025
This emerging concept of pathology-matched mitochondrial therapy will be at the forefront of the World Mitochondria Society (WMS) Annual Meeting, to be held in Berlin this October.
WMS continues to serve as the global platform for shaping the future of mitochondrial research and therapeutic innovation.
Reference
Xiaomeng Lu - Mitochondrial transplantation: adaptive bio-enhancement. Cell Death & Disease. 2025;15(7):309. https://doi.org/10.1038/s41419-025-07643-8




























































