Mitochondria, p53, and the Secret to Aging Gracefully.
As we age, our cells go through stress and damage—especially to their DNA. One of the key proteins that protects us from this damage is p53, often called the “guardian of the genome.” It helps repair broken DNA and prevents damaged cells from turning into cancer.
But this new study, published in Nature Communications, shows that p53 does even more than we thought: it also helps stop inflammation that can build up in aging cells.
The researchers discovered that mitochondria, which are best known for producing energy, play a key role in controlling how p53 works. When mitochondria function well, they support p53 in repairing DNA and stopping the cell from releasing pieces of damaged DNA—called cytoplasmic chromatin fragments (CCFs)—into the rest of the cell. These fragments can trigger chronic inflammation, a common problem in aging and age-related diseases. By keeping DNA inside the nucleus and helping it stay intact, p53 helps reduce this inflammation and keep the cell stable. When mitochondria aren’t working properly, though, p53 can’t do its job as well—leading to more DNA damage, more CCFs, and more inflammation.
This discovery adds an important piece to the puzzle of aging: it shows how mitochondria and p53 work together to protect cells, maintain DNA integrity, and prevent harmful inflammation. It could open new doors for therapies targeting aging, inflammation, and mitochondrial diseases.
More scientific Details :
Using mitochondrial stress models and p53 loss-of-function systems, the authors show that functional p53:
• Promotes DNA damage repair,
• Limits DNA leakage into the cytoplasm,
• Suppresses cGAS-STING-mediated inflammatory signaling,
• And overall contributes to nuclear genome stability in senescent cells.
Notably, the depletion of p53 led to an increase in nuclear envelope instability and accumulation of CCFs, while its presence correlated with reduced senescence-associated secretory phenotype (SASP) expression. These effects were tightly linked to mitochondrial status, suggesting a mitochondria-to-nucleus signaling axis regulating p53 activity.
This work positions p53 as a key integrator of mitochondrial signals to modulate nuclear architecture and inflammatory outcomes during senescence. It highlights potential therapeutic avenues to modulate mitochondrial-p53 interactions in aging-related pathologies and chronic inflammatory conditions.
Immunoengineered Mitochondria for Efficient Therapy
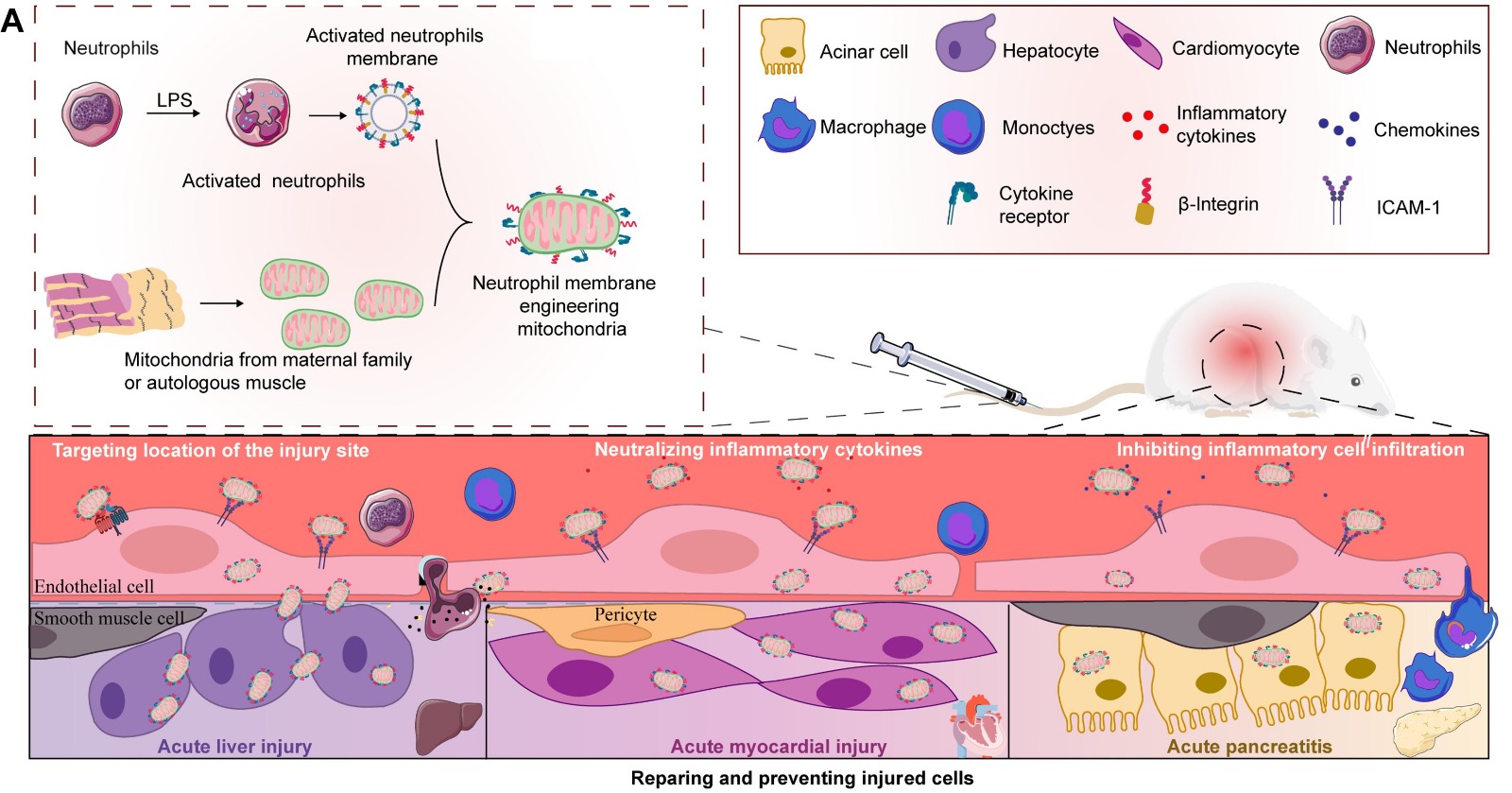
Acute organ injuries (AOIs) remain a significant clinical and public health challenge, often driven by inflammation and mitochondrial dysfunction.
A promising frontier? Mitochondrial transplantation
A recent study in Science Advances takes this concept further, presenting immuno-engineered mitochondria that can evade immune detection — enhancing their integration and therapeutic potential in damaged tissues.
Key Scientific Highlights
• Immunoengineering Concept: Mitochondria are coated or modified to interact more effectively with immune cells and inflamed tissues.
• Improved Uptake: Engineered mitochondria show higher levels of uptake by damaged cells.
• Enhanced Stability: Modified mitochondria remain functional longer within tissues.
• Anti-inflammatory Effect: Treatment reduces inflammation in models of acute injury.
• Effective Repair: Better tissue protection and regeneration were observed.
• Preclinical Models: Proven success in animal models of organ injury (e.g., liver, kidney).
This study demonstrates that immuno-engineered mitochondria can overcome the limitations of traditional mitochondrial therapy. By improving targeting and cellular interaction, they offer a more efficient way to treat AOIs. These findings pave the way for future research into precision mitochondrial medicine, with potential applications in a wide range of diseases involving mitochondrial damage and immune activation.
This innovation could pave the way for more effective interventions for ischemic injuries, kidney damage, and beyond.
What is a Mitochondrial Cybrid? Impact and Strategies by Dr. Alberto Anel

A cybrid (cytoplasmic hybrid) is a laboratory-engineered cell that contains the nuclear DNA (nDNA) from one cell and mitochondrial DNA (mtDNA) from another. Scientists create cybrids to study the role of mitochondria in diseases such as cancer, neurodegenerative disorders, and metabolic conditions.
Why Are Cybrids Important?
Mitochondria, often called the “powerhouses of the cell,” are crucial for energy production and cellular health. However, mutations in mtDNA can contribute to cancer progression, drug resistance, and other diseases.
Cybrids allow researchers to isolate the effects of mitochondrial DNA changes without interference from nuclear DNA. This makes them a powerful tool for biomedical research in understanding how mitochondria impact health and disease.
Applications of Mitochondrial Cybrids
- Disease Modeling: Cybrids help scientists study mitochondrial diseases by isolating the effects of specific mtDNA mutations. For example, they have been used to investigate mitochondrial dysfunction in Alzheimer’s disease.
- Drug Testing: By introducing different mtDNA haplotypes into a consistent nuclear background, cybrids help evaluate how genetic variations affect cellular responses to drugs. This is useful for predicting adverse drug reactions and treatment efficacy.
- Metabolic Research: Cybrids provide insights into how mtDNA variations influence metabolism, particularly in conditions affecting energy production and mitochondrial function.
Significance of Mitochondrial Cybrids
By enabling the study of mtDNA’s role in cellular function without the confounding effects of nuclear DNA, cybrids are crucial for:
✅ Understanding Mitochondrial Diseases: They allow researchers to directly assess how mtDNA mutations contribute to disease pathology.
✅ Personalized Medicine: Cybrid models help predict individual responses to medications based on mitochondrial genetics, paving the way for tailored therapeutic approaches.
✅ Evolutionary Studies: Cybrids offer a platform to study the compatibility and functional impact of introducing mtDNA from different species or populations into a shared nuclear environment.
Dr. Alberto Anel and His Research
Dr. Alberto Anel, a leader at the University of Zaragoza, Spain, is a leading scientist in mitochondrial research, cancer biology, and immunotherapy. His work focuses on how mitochondria influence cancer cell survival, resistance to treatments, and interactions with the immune system.
Implications of Dr. Anel’s Research
✅ Cancer Treatments: Targeting mitochondria could improve chemotherapy and immunotherapy outcomes.
✅ Personalized Medicine: Cybrid models help identify how different mtDNA mutations affect drug response, leading to more precise treatments.
✅ Autoimmune Diseases: Understanding mitochondrial dysfunction could lead to new therapies for immune-related disorders.
The Future of Mitochondrial Cybrid Research
Dr. Anel’s work on mitochondrial cybrids is transforming how we understand cancer, immunity, and mitochondrial diseases. His research could lead to:
✅ More effective treatments
✅ Better patient outcomes
✅ Personalized medicine based on mitochondrial genetics
Mitochondrial cybrids remain a powerful tool in biomedical research, paving the way for innovative therapies and deeper insights into the role of mitochondria in human health.
Image from: A rendering of the exterior of mitochondria. (National Institutes of Health)
NASA’s MitoMars Project Explores Mitochondria Replacement Therapy for Deep Space Missions
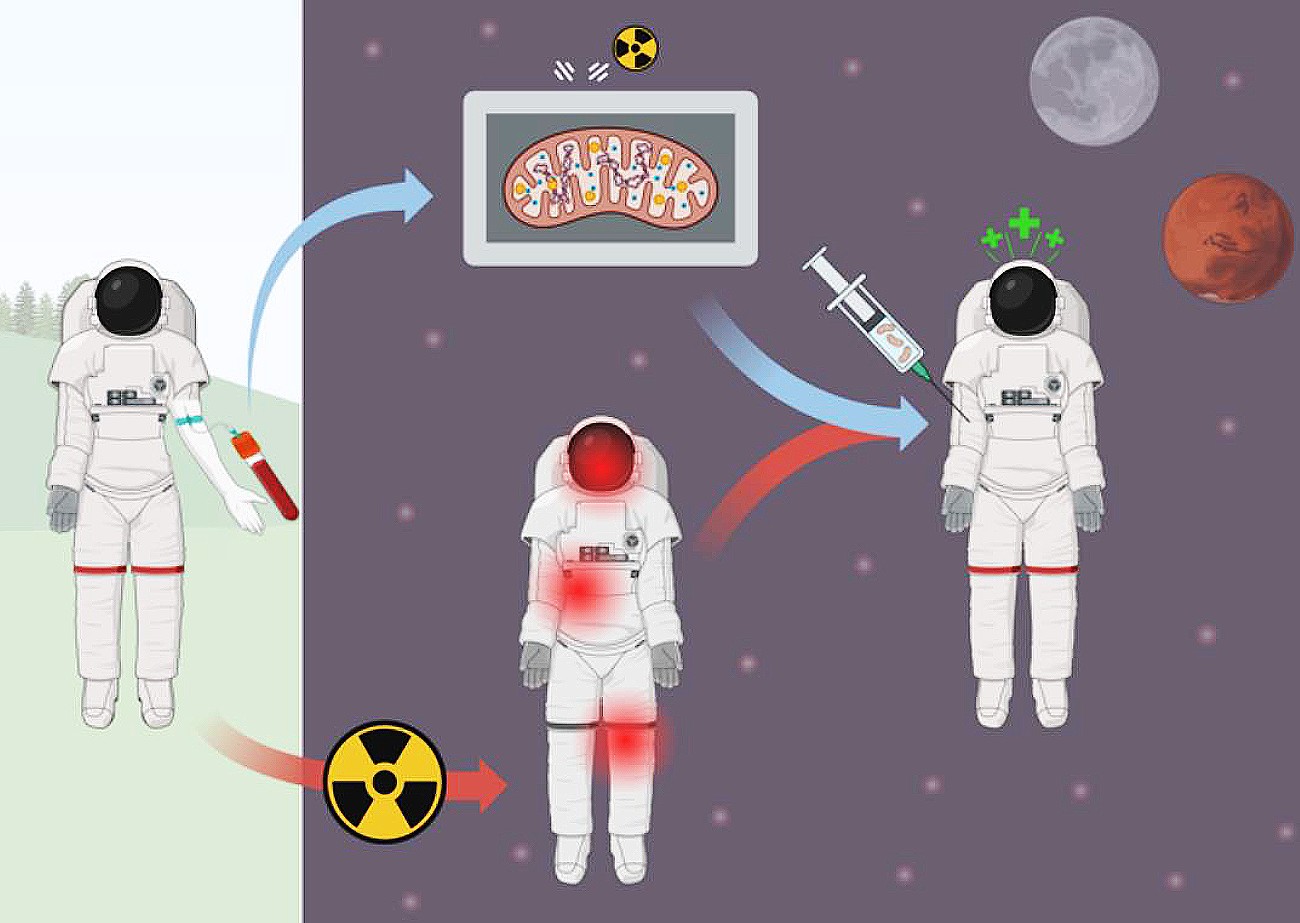
The World Mitochondria Society (WMS) is excited to highlight NASA’s groundbreaking MitoMars project, an initiative focused on targeted mitochondria replacement therapy to enhance astronaut endurance during deep space missions. This research is part of NASA’s 2025 Innovative Advanced Concepts (NIAC) program, which funds pioneering ideas to support human space exploration.
Mitochondria: The Key to Space Endurance
Mitochondria, often referred to as the powerhouses of the cell, play a crucial role in cellular energy production, immune function, and stress resilience. However, exposure to deep-space radiation and microgravity can cause mitochondrial dysfunction, leading to muscle loss, immune suppression, and neurodegeneration. The MitoMars project aims to counteract these effects by developing a novel mitochondria transfer therapy that could restore cellular health and enhance astronaut performance during long-duration missions to the Moon, Mars, and beyond.
Leading the Charge in Space Medicine
The project, led by Robert G. Hinshaw at NASA’s Ames Research Center, is exploring how mitochondria transplantation can rejuvenate human cells after radiation exposure. By using in vitro human cell models, the research team seeks to demonstrate the feasibility of delivering functional mitochondria to damaged tissues, potentially revolutionizing space medicine and human longevity research.
A Breakthrough with Global Implications
While the focus is on space exploration, MitoMars has far-reaching implications for aging, neurodegenerative diseases, and regenerative medicine on Earth. This work aligns with the World Mitochondria Society’s mission to advance research in mitochondrial medicine and promote innovative strategies to combat mitochondrial dysfunction in aging and disease.
An Invitation to Share at WMS in Berlin
We truly hope that Robert G. Hinshaw will join us at the World Mitochondria Society Congress in Berlin to share these exciting findings with the global mitochondria research community. His insights could open new frontiers in understanding how mitochondria restoration can not only support space travel but also improve human health on Earth.
The Extracellular Matrix Integrates Mitochondrial Homeostasis
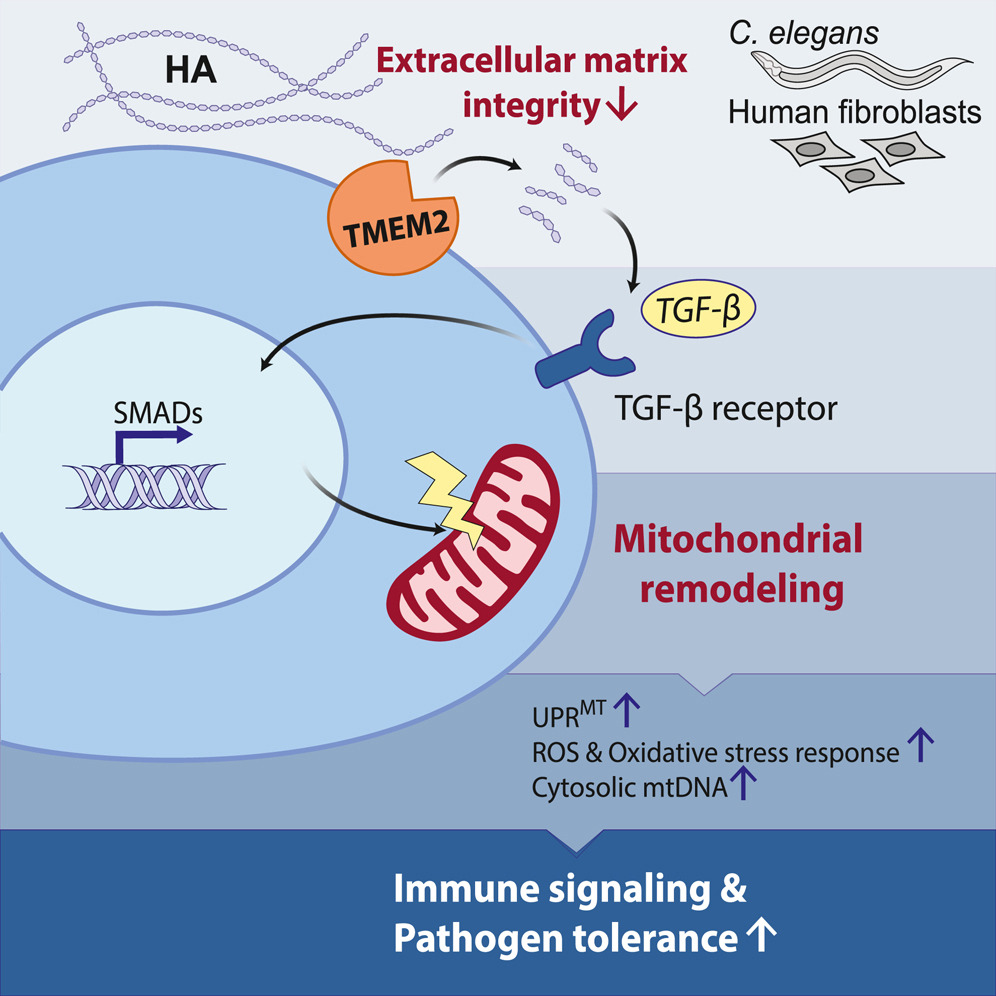
A recent study by Zhang et al. (2024) published in Cell explores a novel connection between the extracellular matrix (ECM) and mitochondrial function. The research demonstrates that ECM remodeling influences mitochondrial homeostasis through an evolutionarily conserved signaling pathway, involving TGF-β activation and mitochondrial stress responses.
Key Findings:
- ECM degradation triggers mitochondrial changes: The breakdown of hyaluronan, a major ECM component, induces mitochondrial fission and stress responses, including the mitochondrial unfolded protein response (UPRMT).
- TGF-β signaling mediates ECM-mitochondria communication: ECM remodeling activates TGF-β signaling, which influences mitochondrial morphology and function.
- Mitochondrial stress enhances immune defense: This ECM-mitochondria pathway plays a role in pathogen defense, suggesting an ancient immune response mechanism that links ECM damage to mitochondrial stress.
- Evolutionary conservation: The study confirms that this pathway is conserved across species, from mammals to C. elegans, reinforcing its fundamental biological importance.
Implications:
This study suggests that ECM integrity is crucial not only for structural support but also for cellular signaling and immune responses. Understanding this ECM-mitochondria crosstalk could open new avenues for research in aging, immune function, and disease treatment.
Image Credit: Hanlin Zhang et al, Journal Cell, Volume 187, Issue 16, 2024
More Articles...
- Mitochondrial Dysfunction Impairs β-Cell Maturation and Function in Diabetes
- Exploiting Mitochondrial Metabolic Vulnerabilities in Glioblastoma: Therapeutic Potential of Mubritinib
- Mitochondria: The Key to Stem Cell Longevity and Anti-Aging Strategies
- How Brain Cell Powerhouses Shape Our Memories: New Research Breakthrough




























































