Cancer’s Energy Hack: Tumor Cells Steal Mitochondria from Nerves to Fuel Metastasis
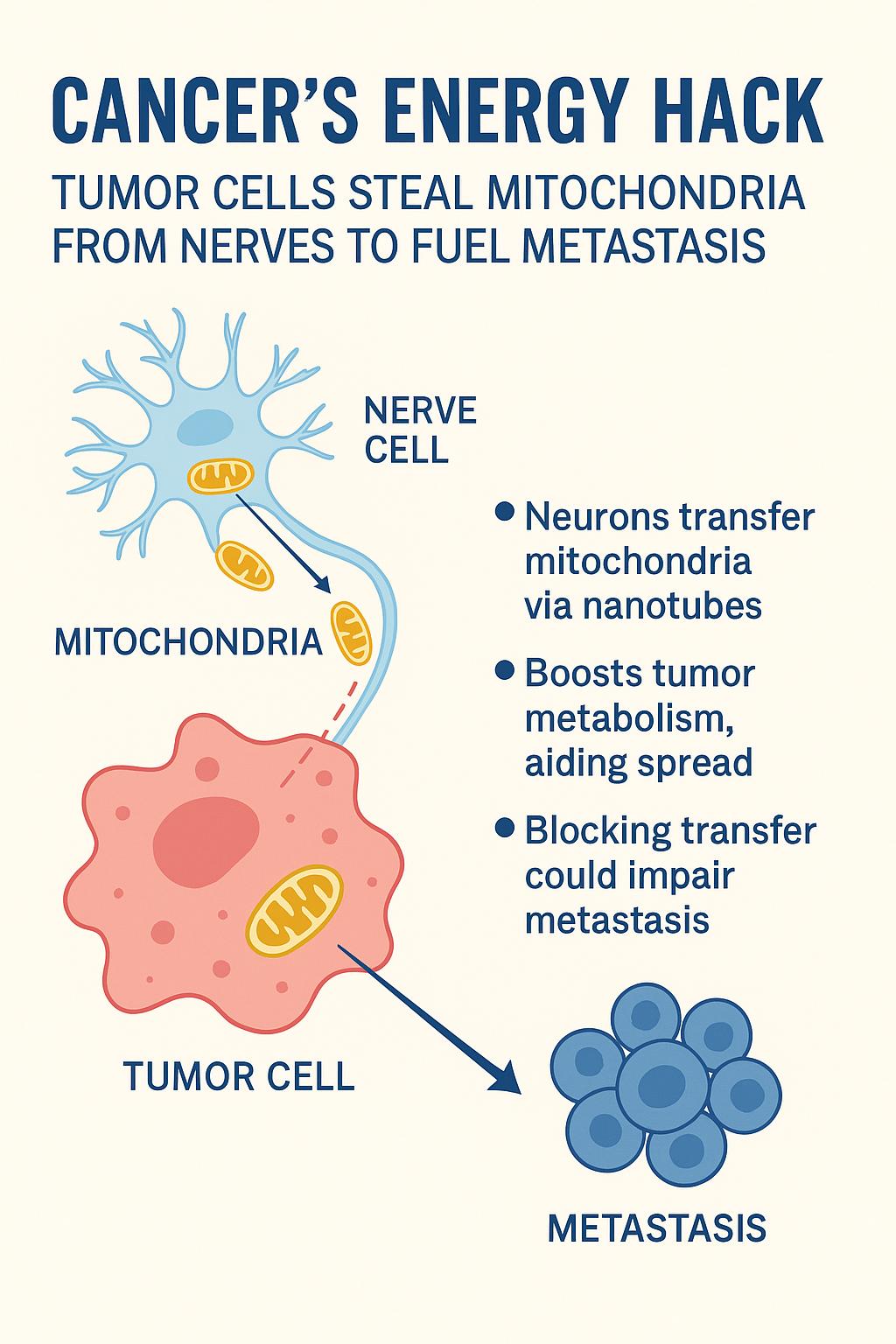
In a striking new study published in Nature, researchers reveal that cancer cells can directly siphon mitochondria from nearby nerve cells — gaining a powerful metabolic edge that helps them spread throughout the body.
A surprising neural alliance
It’s long been known that nerves infiltrate tumors and somehow enhance cancer progression. But the exact mechanism was unclear. This new research, led by Dr. Simon Grelet, shows that neurons surrounding tumors actively produce extra mitochondria and transfer them to cancer cells via nanotubes — thin, tube-like cellular extensions.
These donated mitochondria aren’t damaged or dysfunctional; they’re fully operational, supercharging the cancer cells’ metabolism and helping them survive and proliferate under stress.
A new tool: the MitoTRACER
To track this process, the team developed a genetic sensor called MitoTRACER, which irreversibly labels cancer cells that have absorbed mitochondria from neurons.
In mouse models of breast cancer, these mitochondria-receiving cells were vastly overrepresented in metastatic sites — clear evidence that neuronal mitochondria play a critical role in cancer spread.
Confirmed in human tumors
The findings were validated in human prostate cancer tissue samples. Areas with high nerve density showed more mitochondrial content in nearby cancer cells. Moreover, chemically disrupting the neural supply using botulinum toxin (BoNT/A) reduced the mitochondrial transfer and impaired cancer aggressiveness.
Why it matters :
• A new metabolic weapon: Cancer cells aren’t just reprogramming their own metabolism — they’re outsourcing energy production by stealing power plants from neurons.
• Direct link to metastasis: The mitochondrial boost gives cancer cells the fuel they need to survive oxidative stress and adapt during metastasis.
• A new therapeutic frontier: Targeting the neuron–tumor interaction — by blocking nanotube formation or preventing mitochondrial trafficking — could offer a novel way to slow or stop cancer spread.
More information :
Grelet et al., “Neuronal–tumour cell mitochondrial transfer promotes metastasis,” Nature, 26 June 2025, DOI: 10.1038/s41586-025-09176-8.
A Chimera that Breaches Mitochondria

A Chimera that Breaches Mitochondria
A milestone in mitochondrial biology
A research team from the University Medical Center Göttingen has developed a synthetic chimera that can cross both mitochondrial membranes and selectively block mitochondrial protein synthesis—without altering nuclear DNA.
For the first time, scientists can now disable one mitochondrial protein at a time in living cells, enabling real-time study of bioenergetics, redox balance, and disease pathways.
This innovation bypasses long-standing genetic barriers and opens new possibilities for studying and treating mitochondrial dysfunction in aging, neurodegeneration, and metabolic disease.
Timing of Mitochondrial Dysfunction Found to Shape Lifespan
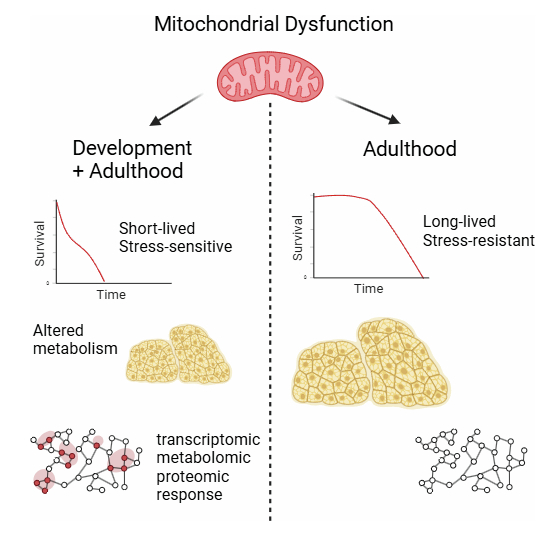
A new study published in EMBO Reports highlights that when mitochondrial complex I (CI) dysfunction occurs dramatically influences lifespan and stress resilience. Using Drosophila models, researchers uncovered striking differences depending on the life stage at which mitochondrial impairment begins:
- Early developmental dysfunction leads to shortened adult lifespan and poor stress resistance.
- Adult-onset dysfunction, despite causing up to a 75% reduction in CI activity, allows flies to live longer and remain stress-resistant.
- Maladaptive biological responses triggered during development—not mere developmental defects—drive the negative outcomes.
- Molecular analyses revealed unique transcriptomic, proteomic, and metabolomic changes in short-lived flies.
The findings suggest that mitochondrial health during early life stages is critical for setting long-term health trajectories. This discovery opens new avenues for developing therapies that target mitochondrial function during key developmental windows to promote healthy aging.
This subject will be talk during the Targeting Mitochondria 2025 congress, which will be held on October 22-24, in Berlin Germany.
Mitochondria Take the Lead: How Metabolism Rewrites Cell Fate
A study led by Scott W. Lowe (Nature, 2025) brings mitochondria into the spotlight — not just as power generators, but as active decision-makers in stem cell fate.
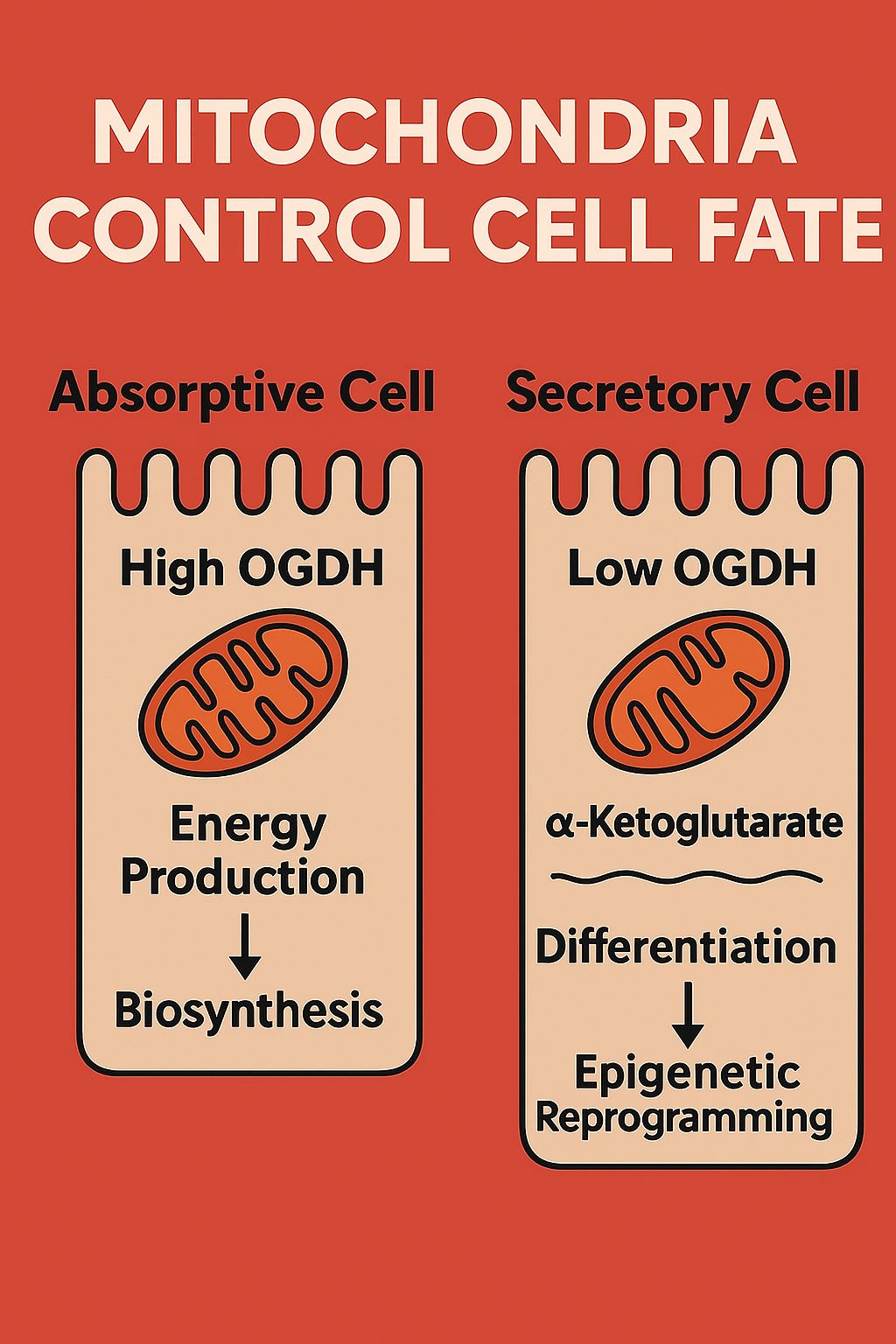
At the heart of the discovery is OGDH, a key enzyme in the mitochondrial TCA cycle. Traditionally seen as a simple metabolic actor, OGDH is now revealed as a master regulator of cell identity in the intestinal epithelium.
- In absorptive cells, high OGDH activity supports energy production and biosynthesis through oxidative phosphorylation, driving cell growth.
- In contrast, low OGDH levels in secretory cells cause an accumulation of α-ketoglutarate (αKG) — a metabolite that acts as a signaling molecule, inducing epigenetic reprogramming and pushing cells toward differentiation.
Using inducible OGDH knockdown in mice, 3D organoid cultures, metabolic tracing, and transcriptomics, the researchers demonstrated that modulating αKG levels can shift stem cell
Mitochondrial Pyruvate Carrier: A Gateway to Future Metabolic Therapies

Structural and mechanistic insights unveil MPC as a strategic target in cancer, liver disease, and mitochondrial medicine. World Mitochondria Society 2025 Copyright
Fifty years after the mitochondrial pyruvate carrier (MPC) was first identified, researchers have now resolved its molecular structure and mechanism of action. In a landmark study published in Science Advances, Sichrovsky et al. (2025) unveiled how this critical mitochondrial complex mediates pyruvate transport and how its inhibition could be leveraged for therapeutic purposes in cancer, metabolic disorders, and more.
What Is the MPC and Why Is It Important?
The mitochondrial pyruvate carrier (MPC) is a protein complex located in the inner mitochondrial membrane. It enables the import of pyruvate, a central metabolite derived from glycolysis, into mitochondria where it fuels the TCA cycle and ATP production. This process is fundamental to energy metabolism, redox regulation, and biosynthesis.
Until now, the exact structure and working mechanism of the MPC had remained elusive, limiting our ability to design targeted drugs.
Major Discoveries of the Study by Prof. Edmund Kunji and his teams
Molecular Structure of MPC:
The authors used cryo-electron microscopy to capture the architecture of the human MPC complex. They discovered that MPC forms a heterodimeric transport unit (MPC1/MPC2), creating a selective channel that guides pyruvate across the inner mitochondrial membrane.
Mechanism of Transport and Inhibition:
The study revealed how small-molecule inhibitors bind to the MPC complex and block its function, offering a blueprint for drug development. Structural analysis pinpointed specific binding sites that explain both transport dynamics and inhibition sensitivity.
Conserved Functionality:
Evolutionary conservation of the MPC mechanism across species (including yeast and human) underscores its universal biological role in cellular energy homeostasis.
Therapeutic Implications
Cancer:
Some tumors overexpress MPC to fuel high mitochondrial activity. MPC inhibitors could starve these cells of essential metabolites, selectively disrupting their growth.
Metabolic Diseases:
In conditions like non-alcoholic fatty liver disease (NAFLD), blocking MPC forces hepatocytes to burn fat instead of relying on glucose, leading to reduced liver fat accumulation.
Regenerative Medicine & Hair Growth:
MPC inhibition has been shown to stimulate lactate production, which may promote hair follicle cell activation, opening potential new treatments for alopecia.
Mitochondrial Dysfunction & Neurodegeneration:
Targeting MPC may allow modulation of energy metabolism in neurodegenerative and mitochondrial diseases, where ATP production and redox balance are impaired.
Broader Impact
Drug Development:
The structural elucidation of MPC provides a molecular framework for designing selective modulators, setting the stage for new classes of metabolic drugs.
Precision Medicine:
Understanding individual differences in MPC structure/function may lead to personalized metabolic therapies tailored to genetic or disease-specific metabolic profiles.
Synthetic Biology & Bioenergetics:
The detailed MPC model can inform the engineering of customized metabolic pathways, supporting advances in synthetic biology, cell therapies, and biotechnology.
Statement of Prof. Marvin Edeas and Prof. Volkmar Weissig, chairman of World Mitochondria Society, "The study by Sichrovsky et al. marks a strategic breakthrough in the field of mitochondrial biology and cellular metabolism. By revealing the detailed structure and transport mechanism of the mitochondrial pyruvate carrier (MPC), the researchers have clarified how a key metabolic gatekeeper functions at the molecular level. This discovery is important because it gives scientists the precise blueprint needed to design drugs that selectively modulate mitochondrial metabolism. Such interventions could target cancer cells that rely on mitochondrial energy, help treat liver conditions by shifting how cells use nutrients, and even promote regeneration in other tissues."
Overall, this work transforms the MPC from a poorly understood protein into a strategic therapeutic target. It opens new possibilities for developing mitochondria-targeted treatments that are safer, more effective, and tailored to the metabolic needs of each disease.
Prof. Edmund Kunji from the University of Cambridge will give a major talk entitled Targeting mitochondrial pyruvate carrier: impact on future metabolic therapies, during the Targeting Mitochondria 2025 Congress, which will be held on October 22-24, in Berlin Germany.
Reference:
Sichrovsky, M., Lacabanne, D., Ruprecht, J.J., Rana, J.J., et al. (2025). Molecular basis of pyruvate transport and inhibition of the human mitochondrial pyruvate carrier. Science Advances, 18 April 2025. DOI: 10.1126/sciadv.adw1489
More Articles...
- Dnmt3a mutation enhances mitochondrial function in blood stem cells, leading to clonal expansion and increased disease risk
- Zika Virus NS1 Hijacks Mitochondria Through Tunneling Nanotubes for Immune Evasion
- How Mitochondria Organize Their Powerhouse Machinery for Optimal Performance
- Revolutionary Breakthrough in Mitochondrial Production Offers Hope for Degenerative Diseases




























































