New Treatment Shows Promise for POLG-Related Mitochondrial Disorders
A clinical trial led by the Research Institute of the McGill University Health Centre (RI-MUHC) has identified Deoxycytidine/Deoxythymidine Combination Therapy as a safe and potentially effective treatment for POLG-related mitochondrial disorders. These disorders cause severe neurological decline, with patients typically surviving only five months after symptom onset. The preliminary results, published in eClinicalMedicine, were largely funded by the Liam Foundation, established after a patient’s diagnosis at the Montreal Children’s Hospital (MCH).
Dr. Kenneth Myers, a pediatric neurologist at MCH, noted, “Our study offers new hope, transforming what was once a death sentence into a chance for a better life. While not a cure, the treatment has significantly improved patients' conditions”.
Understanding the Condition
Mitochondrial diseases, affecting one in 5,000 people, result from dysfunctional mitochondria, the energy-producing parts of cells. In POLG-related disorders, mutations in the POLG gene reduce mitochondrial DNA (mtDNA), leading to seizures, vision loss, muscle issues, nerve damage, developmental delays, and liver failure. The therapy aims to replenish the mtDNA, enhancing mitochondrial function.
After six months, patients showed improved scores on the Newcastle Mitochondrial Disease Scale and lower levels of GDF-15, a marker of mitochondrial dysfunction. Caregivers reported better energy, motor skills, cognition, and communication. No serious side effects were observed.
Dr. Myers highlighted, “Many patients regress dramatically after infections or other triggers. This treatment supplies the mitochondrial DNA they need to function normally.”
Expanding the Trial
The trial included 10 children and adolescents with POLG mutations from the US, Brazil, and India. They received the treatment for six months, with some continuing for 24 months due to significant improvements. Another 14 patients have joined, and a follow-up study on long-term effects is underway.
Liam’s Story: From Despair to Hope
Liam, a ten-year-old with POLG-related mitochondrial disease, began having seizures in 2019. His father, Kevin Reason, started the Liam Foundation after learning about the potential of Deoxycytidine/Deoxythymidine. “Liam is now walking, communicating, and smiling. This treatment gives us hope and vital time to find a cure”, Kevin said.
Liam was the first North American patient in the trial. Thanks to the Liam Foundation and other supporters, 23 more POLG patients have since enrolled.
Prof. Volkmar Weissig's Interview: Is it time for mitochondria to take centre stage?
It is a great pleasure to announce that the interview of Professor Volkmar Weissig, president of the World Mitochondria Society, has been published in Presciber Journal.
In his interview, Prof. Weissig discusses the profound impact of mitochondrial dysfunction on human health. He highlights a landmark case from the 1950s that first linked malfunctioning mitochondria to human disease, spurring extensive research over subsequent decades.
"There may not be any disease that does not, in one way or another, involve dysfunctional mitochondria".
Prof. Weissig elaborates on the widespread prevalence of primary mitochondrial disorders (PMDs) and the role of dysfunctional mitochondria in common diseases such as diabetes, cardiovascular disease, cancer, and Alzheimer's disease. He emphasizes the critical importance of mitochondria in cellular energy production and their involvement in numerous physiological and pathological processes.
The interview also highlights the historical evolution of mitochondrial research, from their identification as the "powerhouse of the cell" to their recent recognition as crucial players in immunity and cellular signaling. Prof. Weissig shares insights into innovative therapeutic approaches, including gene therapy and mitochondrial transplantation, that hold promise for treating mitochondrial diseases.
This interview sheds light on the essential role of mitochondria in health and disease and highlights ongoing efforts to translate mitochondrial research into clinical therapies, aiming to improve the quality of life for patients with mitochondrial disorders.
Read the full interview: https://doi.org/10.1002/psb.2128.
Mitochondrial DNA: A Key Player in Cell Death and Inflammation
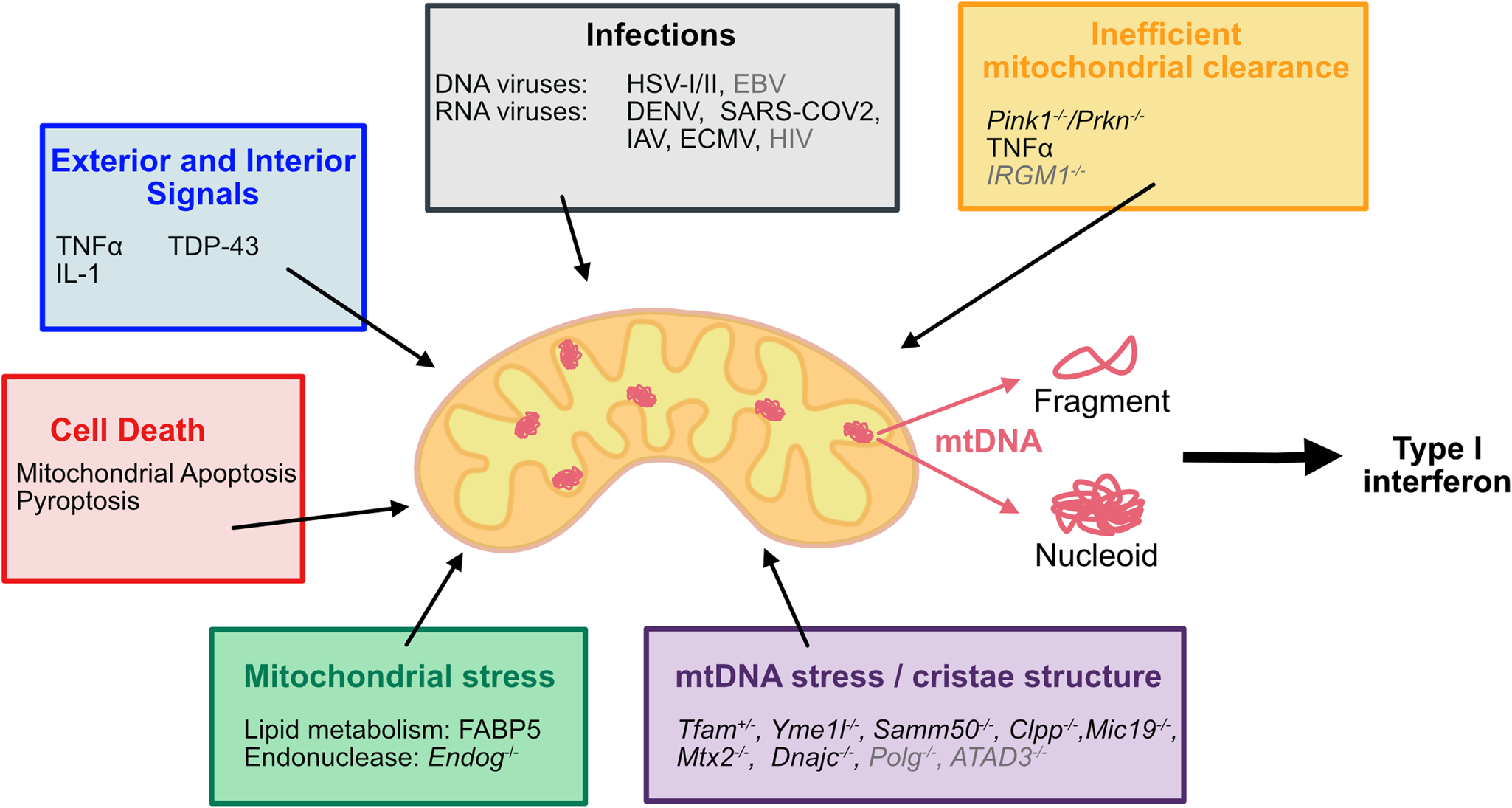
Overview of factors inducing mtDNA release
Cytosolic DNA is recognized by the innate immune system as a potential threat. During apoptotic cell death, mitochondrial DNA (mtDNA) release activates the DNA sensor cyclic GMP–AMP synthase (cGAS) to promote a pro-inflammatory type I interferon response. This inflammation can engage anti-tumor immunity, offering a potential avenue for cancer therapy.
Stephen W.G. Tait explained that various studies have described mtDNA leakage independent of cell death, triggered by pathogenic infections, changes in mtDNA packaging, mtDNA stress, or reduced mitochondrial clearance. While the interferon response in these scenarios can be beneficial, it may also contribute to disease phenotypes. Understanding the cues and factors inducing mtDNA leakage into the cytosol during cell death and beyond is crucial to purposely promote (e.g., in cancer, infections) or hinder (e.g., mtDNA-associated disease phenotypes) mtDNA release pharmacologically.
During apoptosis, mtDNA is released upon mitochondrial outer membrane permeabilization (MOMP) in caspase-deficient conditions. The cytosolic mtDNA activates the DNA sensor cGAS, inducing an NFκB and type I interferon response, thereby promoting inflammation. Beyond apoptosis, other cues such as mitochondrial stress, mtDNA stress, and pathogenic infections induce mtDNA release through distinct mechanisms, causing inflammation via activation of DNA sensors (cGAS) and receptors (NLRP3, AIM2, or TLR9).
To date, mtDNA release has been observed in a variety of systems and circumstances, resulting in inflammatory gene expression. However, the mechanistic insight into how mtDNA reaches the cytosol and how its recognition is regulated remains ambiguous and requires further research. Moreover, understanding the impact of mtDNA-driven inflammation in health and disease is vital for pharmacological intervention and necessitates in-depth knowledge of the pathway.
Dr. Stephen Tait will present his latest findings on mitochondria and inflammation at the Targeting Mitochondria 2024 conference on October 29-31 in Berlin.
Bridging In Vitro and In Vivo for Mitochondrial Transplantation in Acute Diseases
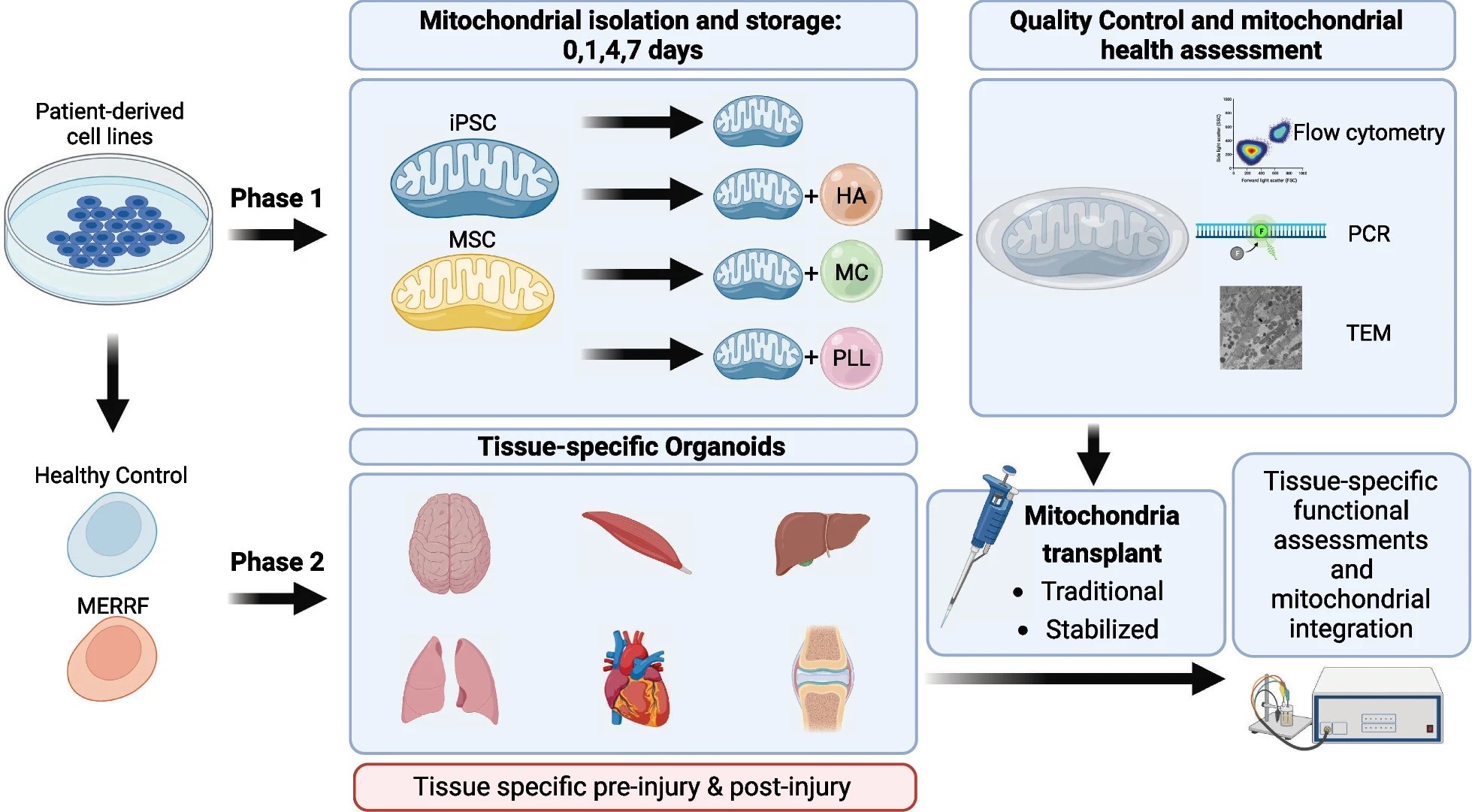
Mitochondrial transplantation and transfer are being explored as therapeutic options in acute and chronic diseases to restore cellular function in injured tissues. Current clinical applications focus on the delivery of autologous mitochondria to limit immune responses and rejection.
A Mitochondrial Transplant Convergent Working Group (CWG) was recently convened to explore three key issues limiting clinical translation:
- Storage of mitochondria
- Biomaterials to enhance mitochondrial uptake
- Dynamic models to mimic the recipient tissue environment
Anna C. Andreazza and her team presented a summary of CWG conclusions related to these three issues and provided an overview of pre-clinical studies aimed at building a more robust toolkit for translational trials.
While mitochondrial transplants show promise in animal and early clinical trials, many questions remain. The CWG identified priorities to accelerate progress, including the need for improved storage and delivery of isolated mitochondria, and accurate in vitro models to mimic human tissue complexity.
A research plan was developed to evaluate the stabilization and short-term storage of mitochondria encapsulated in hyaluronic acid, methyl cellulose, and poly(L-lysine). Storing isolated mitochondria enables comprehensive quality control, ensuring healthy and pure transplants, reducing adverse events, and allowing for procedures requiring multiple transplants without repeated isolations.
Organ-on-a-chip models for brain, cardiac, muscle, joint, lung, and liver tissues will be used to evaluate mitochondrial transplants in acute and chronic disease states. This approach allows accurate assessment of clinical applicability and therapeutic mechanisms in vitro, advancing mitochondrial transplant translation across various diseases.
Anna C. Andreazza will be a speaker at the 15th WMS Annual Meeting this October in Berlin. She will discuss advances in mood disorders and mitochondrial-organ transplantation.
Image Credits: Bodenstein, D.F., Siebiger, G., Zhao, Y. et al. Stem Cell Res Ther15, 157 (2024)
Mitochondrial Extracellular Vesicles: Promising Therapeutic Strategy for Neurological Diseases
A recent paper by Dr. Stefano Pluchino, University of Cambridge and invited speaker at Targeting Mitochondria 2024, in collaboration with colleagues from the University of Duisburg-Essen, highlights the transformative potential of extracellular vesicles (EVs) in treating neurological diseases, providing a comprehensive overview of their versatile mechanisms and therapeutic promise.
Extracellular vesicles (EVs) are naturally occurring membrane particles that play a crucial role in cellular communication. Unlike traditional pharmacological treatments that target specific signaling pathways, EVs offer a multifaceted approach by modulating complex disease processes through various effectors. This unique capability positions EVs as powerful agents in promoting brain tissue recovery.
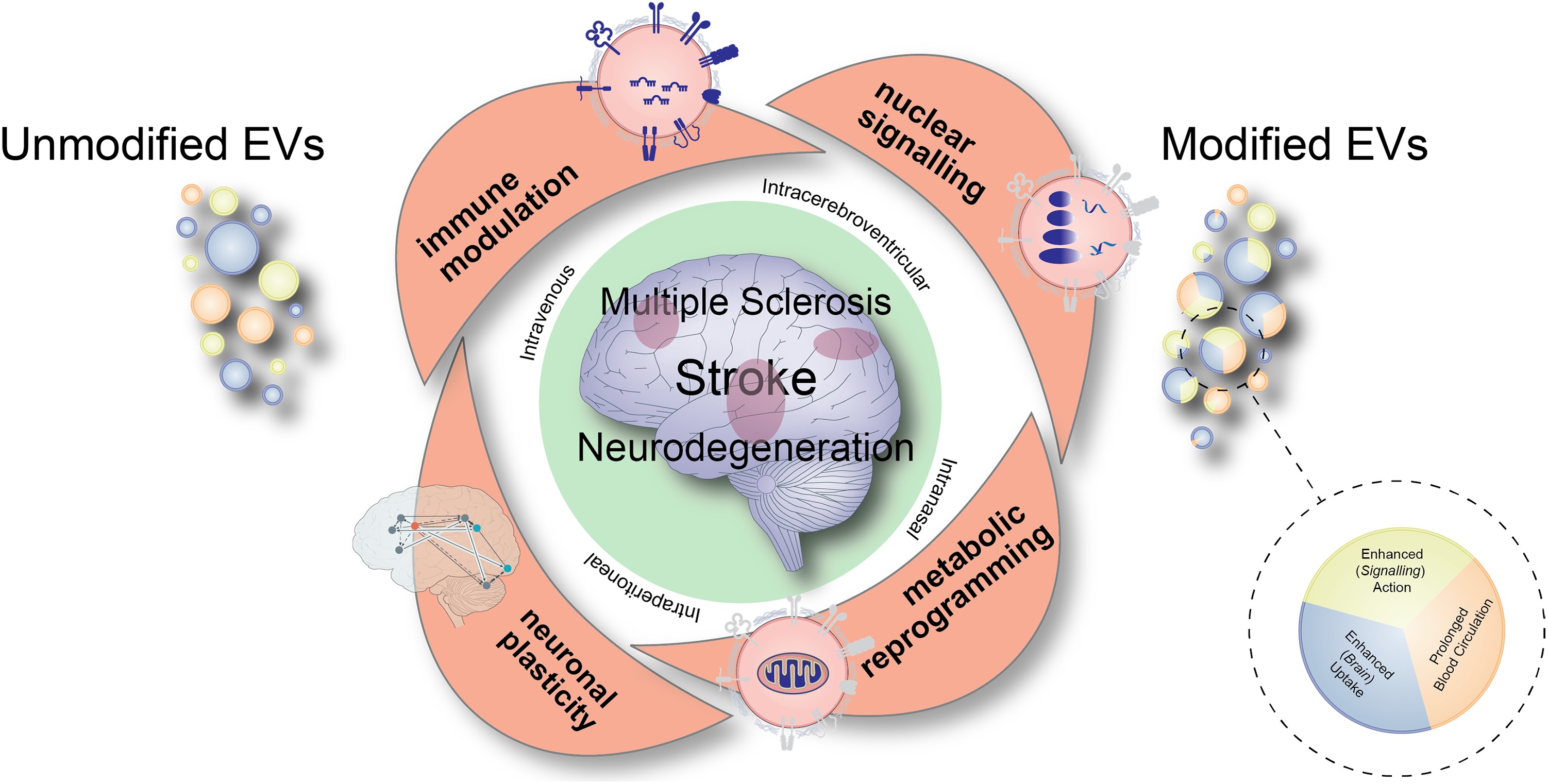
Cartoon summarizing major modes of actions of extracellular vesicles that are therapeutically administered via different routes in diverse disease conditions including stroke, multiple sclerosis or neurodegenerative diseases. The different modes of action, which comprise immune modulation, nuclear signalling, metabolic reprogramming and promotion of neuronal plasticity, synergistically contribute to the recovery-promoting effects of extracellular vesicles (EVs). For therapeutic purposes, unmodified EVs are currently evaluated, as well as EVs that have genetically been modified enabling prolonged EV circulation in the blood, enhanced brain uptake or enhanced signalling action, respectively.
The study reveals that EVs derived from different cellular sources can induce significant therapeutic responses in experimental models of neurological diseases. When administered in vivo, EV-based treatments have shown remarkable effects on immune responses, cell metabolism, and neuronal plasticity. This multimodal influence on neuroimmune networks enables EVs to modulate disease processes synergistically and context-dependently, leading to profound neurological recovery.
Dr. Pluchino and his team provide a detailed exploration of how EVs identify cellular targets and transmit signals to recipient cells. They highlight the relevance of these mechanisms in key neurological conditions such as stroke, multiple sclerosis, and neurodegenerative diseases. The review discusses critical pathways that warrant further investigation in specific disease contexts, showcasing the potential of EVs to revolutionize treatment approaches.
The paper also addresses important considerations regarding EV biodistribution and the genetic engineering strategies designed to enhance brain uptake and signaling. These advances aim to maximize the therapeutic efficacy of EVs, ensuring targeted delivery and robust clinical outcomes.
The researchers also outline the future clinical applications of EVs and propose essential information required for the successful translation of EV-based therapies into clinical trials. This forward-looking perspective highlightses the potential of EVs as next-generation therapeutics for brain diseases, emphasizing the need for rigorous scientific discussion and clinical validation.
Dr. Pluchino will be joining Targeting Mitochondria 2024 this October to develop this topic with a talk entitled "Mitochondrial Extracellular Vesicles: Orchestrating Brain Plasticity and Recovery".
More Articles...
- Exploring Age-Related Changes in Mitochondrial Structure and Function
- HDL-C and Ferritin: Key Metabolic Indicators of Long COVID-19 Severity Revealed, Opening New Avenues for Treatment Strategies
- Transforming Transplantation: Real-Time Monitoring of Mitochondrial Health During Organ Perfusion
- Mitochondria and Powering the Mind: Secrets of Synaptic Energy with VAP




























































