Military Medicine and Mitochondria: A Strong Interest in Targeted Strategies and Therapeutics

During the 15th Annual Meeting of the World Mitochondria Society (WMS), taking place in Berlin, Germany, groundbreaking research on mitochondria-targeted medicine in military settings will be highlighted. This research, particularly focused on Traumatic Brain Injury (TBI) and polytrauma, is of significant interest due to the high incidence of such injuries in combat, where multiple organs are affected by mechanical trauma, thermal injuries, or exposure to harmful agents such as chemical, biological, radiological, or nuclear materials.
Mitochondria-targeted medicine has emerged as a crucial area for military medicine, especially for addressing TBI. Research from the Walter Reed Army Institute of Research has emphasized the essential role of mitochondrial function in the acute phase following TBI. The research shows that TBI leads to bioenergetic failure and disruptions in calcium and redox balance, which are critical for recovery. The findings suggest that therapies aimed at restoring mitochondrial function may serve as an effective neuroprotective approach.
Moreover, the detection of mitochondria-specific markers in biofluids presents exciting opportunities for advancing diagnostics and therapeutics for TBI. These discoveries could lead to more accurate diagnoses and the development of personalized therapies that enhance recovery for military personnel.
 "The potential to target mitochondrial function in TBI treatment opens new doors for more effective interventions, offering hope for improved outcomes for service members affected by combat injuries", said Dr. Jignesh Pandya, Director of Brain Trauma Bioenergetics, Metabolism & Neurotherapeutics Development and leading researcher on the project.
"The potential to target mitochondrial function in TBI treatment opens new doors for more effective interventions, offering hope for improved outcomes for service members affected by combat injuries", said Dr. Jignesh Pandya, Director of Brain Trauma Bioenergetics, Metabolism & Neurotherapeutics Development and leading researcher on the project.
These findings, which underline the military's growing focus on mitochondria-centric therapeutic strategies, will be a focal point of discussion at the WMS Annual Meeting, attracting attention from the global scientific and medical community.
More about Dr. Pandya's talk at Targeting Mitochondria 2024.
About the World Mitochondria Society (WMS)
The World Mitochondria Society (WMS) is an international organization dedicated to advancing research on mitochondria and their role in health and disease. The society promotes global collaboration among scientists and medical professionals to accelerate breakthroughs in mitochondrial medicine, diagnostics, and therapies. The WMS Annual Meeting serves as a platform for presenting the latest scientific advancements and fostering dialogue on the future of mitochondrial research.
For more information on this research and its implications for military medicine, please contact the WMS (mitochondria[at]wms-site.com).
Speakers Line-up and other topics.
Abstracts to be presented
State of the Science Mitochondria Specific-Targets, Therapeutics and Biomarker Investigations following Traumatic Brain Injury in the US Military
Jignesh D. Pandya, Walter Reed Army Institute of Research, USA
Access Abstract
Variation in Mitochondrial Functions across Vital Organs and Brain Sub-Regions in a Swine Model: A Novel Reference Targets forTBI and Polytrauma
Anke H. Scultetus, Walter Reed Army Institute of Research, USA
Access Abstract
Enhancing T Cell Antitumor Activity through Mitochondrial Transfer
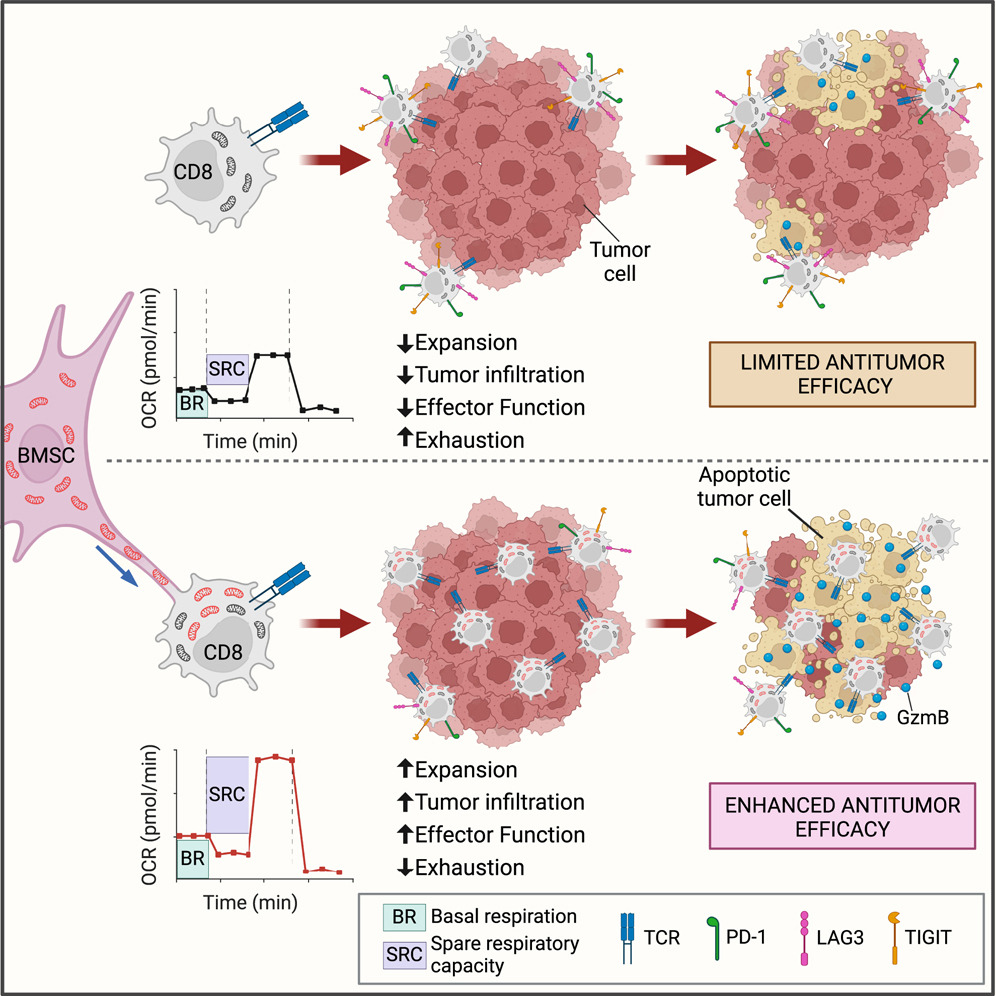
The research demonstrated that bone marrow stromal cells (BMSCs) transfer mitochondria to T cells through intercellular nanotube connections, significantly increasing T cell mitochondrial mass and respiration. This process, requiring Talin 2 on both donor and recipient cells, improves the metabolic function of T cells, enabling them to infiltrate tumors more effectively and show fewer signs of exhaustion.
By utilizing this intercellular mitochondrial transfer, the study offers a new approach to overcoming T cell exhaustion, a critical challenge in immunotherapy. This breakthrough in organelle medicine could lead to the development of next-generation cell therapies with enhanced efficacy against cancer.
The latest innovations in mitochondrial research and mitochondrial transfer, including findings like this, will be discussed at the 15th World Congress on Targeting Mitochondria, taking place from October 29-31, 2024, in Berlin, Germany.Image Credits: Graphical Abstract Baldwin, Jeremy G. et al. Cell (2024)
Mitochondrial DNA in Our Brains Could Be Shortening Lifespan, New Study Reveals
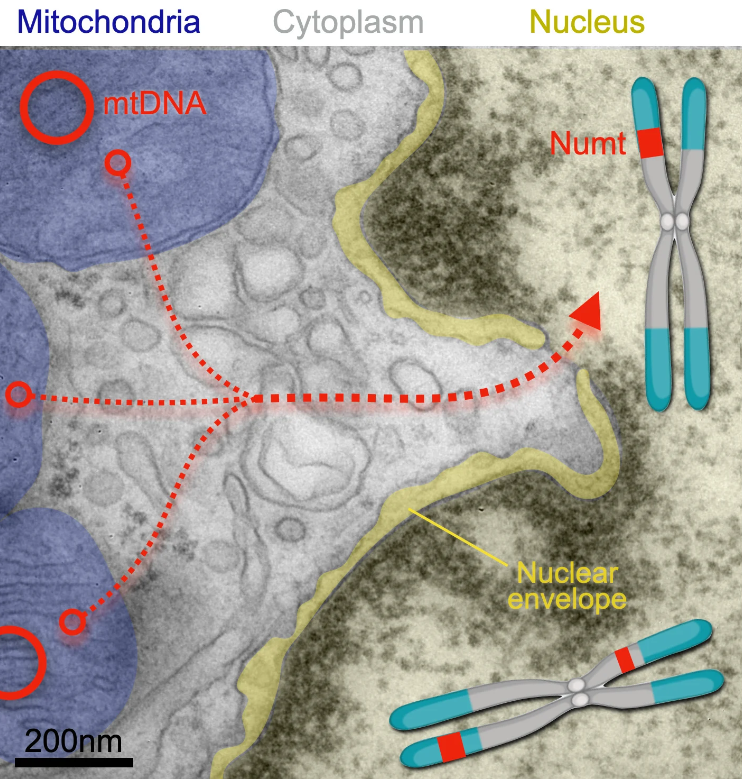
Mitochondria release segments of mitochondrial DNA that can travel through pores of the nucleus and integrate into a cell’s chromosomes (where the insertions are called NUMTs, for nuclear mitochochondrial segments). A new study has found that nuclear mitochondrial DNA insertion—once thought rare—happens in the human brain likely several times over during a person’s lifespan. Credit: Martin Picard laboratory at Columbia University Vagelos College of Physicians and Surgeons
Mitochondria: Key Players in Autism Spectrum Disorder?
Recent review from the Hebrew University of Jerusalem highlighted the multifaceted role of mitochondria in Autism Spectrum Disorder (ASD), suggesting that mitochondrial dysfunction may significantly contribute to the development and pathology of this neurodevelopmental disorder. Mitochondria, essential for producing the aerobic energy necessary for brain function, have been found to exhibit abnormalities in individuals with ASD, which could profoundly impact brain development and function.
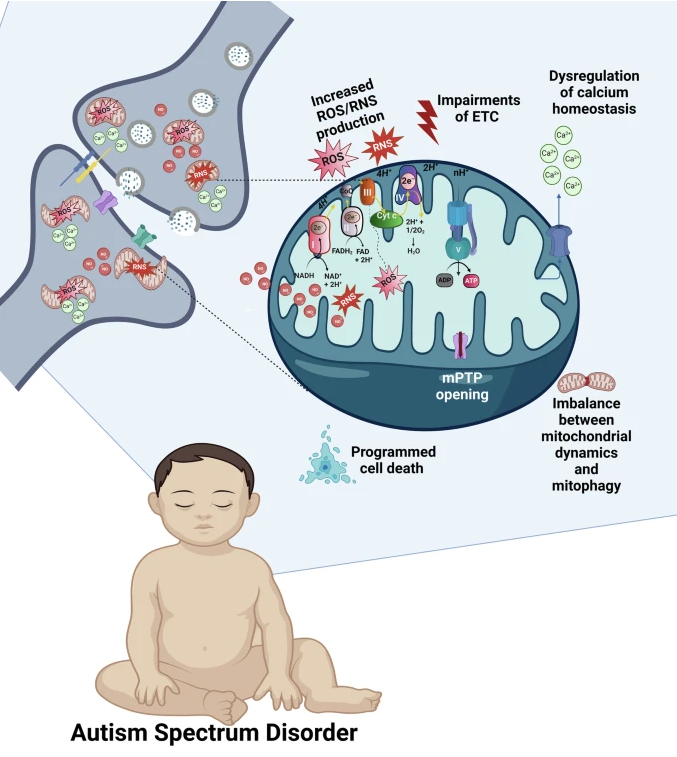
The brain displays high mitochondrial content, particularly in the synapses (shown in the upper left part of the figure). Increased mitochondrial levels of ROS, NO, and RNS, ETC impairments leading to the breakdown of OXPHOS and ATP production, dysregulation of the mitochondrial Ca2+cycling, imbalance between mitochondrial dynamics and mitophagy, prolonged opening of the mPTP, and activation of various mitochondria-related programmed cell death pathways, all contribute to the synaptic dysfunction and ASD. OXPHOS complexes are shown on the upper semisphere of the mitochondrion: I, NADH dehydrogenase; II, succinate dehydrogenase; III, ubiquinone cytochrome c oxidoreductase; IV, cytochrome c, cytochrome oxidase; and V, ATP synthase. Complexes I-IV belong to ETC.
ASD is associated with a variety of mitochondrial abnormalities, including impaired respiratory function, disrupted calcium (Ca2+) cycling, altered production of reactive oxygen and nitrogen species (ROS/RNS), and issues with the opening of the mitochondrial permeability transition pore (mPTP). These dysfunctions can lead to the activation of various mechanisms of programmed cell death, an imbalance in mitochondrial fusion, fission, and autophagy processes, and disturbances in synaptogenesis and synaptic transmission,all of which affect brain development and may result in behavioral deficits.
The importance of mitochondria in ASD cannot be overstated. These organelles are crucial for numerous cellular functions and can be affected by different pathogenic factors, which may explain the similarity in behavioral phenotypes seen in ASD cases of varying origins. Synapses, along with mitochondria, are considered key players in the molecular mechanisms related to ASD. The convergence of various neurodevelopmental pathological processes on synapses may partly explain the behavioral similarities observed in individuals across the autism spectrum.
Interestingly, as the recent review discusses, synaptic abnormalities are closely tied to mitochondrial dysfunction in ASD, suggesting that mitochondria-associated synaptic disturbances could present robust therapeutic targets that have yet to be explored. Although there are still many unknowns in the mitochondria-related mechanisms of autism, understanding these “blank spots” could pave the way for novel and effective treatments for ASD. This is particularly significant given the increasing prevalence of ASD and the current lack of effective pharmacological treatments.
Figure credits: Khaliulin, I., Hamoudi, W. & Amal, H. Mol Psychiatry (2024).
Advances in Mitochondrial Modulation: How Infrared Light is Changing Brain Injury Recovery
A recent study has spotlighted the transformative role of near-infrared (NIR) light in improving mitochondrial dynamics and quality control, offering new hope for brain injury recovery following cardiac arrest. Dr. Maik Hüttemann, Wayne State University (USA) and active member of the WMS Scientific Board will join Targeting Mitochondria 2024 Congress in Berlin, where he will delve deeper into these findings and discuss the advances in infrared light treatment.
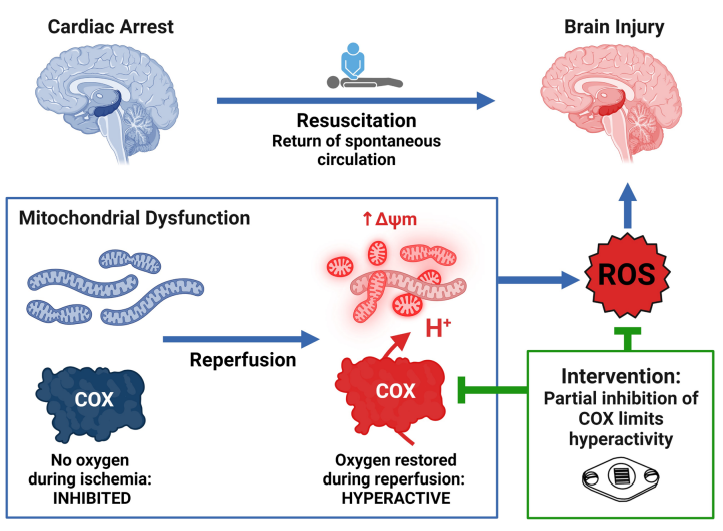
Brain injury remains a significant challenge following cardiac arrest, with mitochondrial dysfunction playing a pivotal role in exacerbating neurological damage. The study investigates how targeting mitochondrial dysfunction with near-infrared light (NIR) wavelengths can mitigate brain injury following cardiac arrest. By employing various models, including isolated porcine brain cytochrome c oxidase (COX), primary mouse neurons, and large animal models, the research provides new insights into NIR-induced mitochondrial modulation.
The research demonstrates that NIR treatment reduces COX activity in an intensity-dependent manner, achieving a controlled modulation of mitochondrial function. This approach results in a moderate reduction of enzyme activity without complete inhibition. Additionally, in neuronal cells, NIR therapy has been shown to decrease mitochondrial swelling and enhance mitophagy, indicating improved mitochondrial health and quality control.
Practical application of NIR therapy has also been investigated. In anesthetized pigs, NIR was found to penetrate deep into the brain with minimal tissue heating, making it a feasible noninvasive treatment option. Moreover, in a model of out-of-hospital cardiac arrest, NIR treatment applied during resuscitation resulted in significantly improved neurological outcomes and reduced brain injury.
The study concludes that NIR effectively modulates mitochondrial function, enhancing mitochondrial dynamics and quality control after ischemia/reperfusion. This noninvasive technique offers promising potential for improving neurological recovery in patients resuscitated from cardiac arrest.
Join Dr. Hüttemann at the Targeting Mitochondria 2024 Congress in Berlin to know more about these findings and explore the future of mitochondria and photomedicine.
Image credits: Wider, J.M., Gruley, E., Morse, P.T. et al. Modulation of mitochondrial function with near-infrared light reduces brain injury in a translational model of cardiac arrest. Crit Care27, 491 (2023).




























































