New study outlines how brain cancer cells take mitochondria from healthy cells to grow and survive
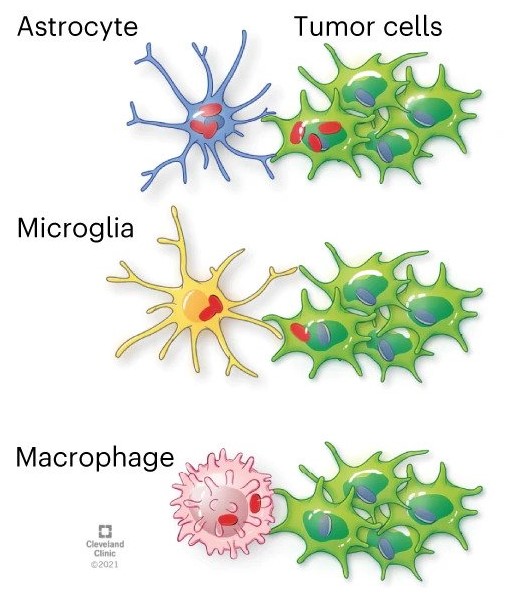
GBM cells acquire mitochondria from astrocytes
Glioblastoma cancer cells use mitochondria from the central nervous system to grow and form more aggressive tumors, according to new Cleveland Clinic-led findings published in Nature Cancer.
The research showed that it is common for healthy astrocytes – a type of glial cell with important functions in the central nervous system – to transfer the energy-producing organelles to glioblastoma cancer cells. When this process happens, it makes the cancer more deadly and the tumors more likely to grow. Researchers found that acquiring mitochondria boosted energy production and amplified cancer stem cells – cells with properties that already make cancer more difficult to treat.
"Defining the complex interactions glioblastoma cells have with the brain and nervous system is critical for developing new treatments for this highly aggressive form of brain cancer" says Justin Lathia, PhD, staff in Cardiovascular & Metabolic Sciences and the Melvin H. Burkhardt Endowed Chair for Neuro-Oncology Clinical Research. “We knew that this type of transfer was theoretically possible, but we didn’t know how relevant and dangerous it was in brain tumors.”
Cancers, including glioblastoma, are resilient in part because of resources in the environment, capitalizing on the body’s natural defenses to protect cancer cells. By determining how cancer cells interact with healthy cells to survive, researchers can design new treatments to block cancer from growing or resisting treatment.
This study investigated mitochondria transfer in glioblastoma, the most common and deadly type of primary brain cancer. The paper’s first co-authors are Dionysios C. Watson, MD, PhD, and Defne Bayik, PhD, both previously of Cleveland Clinic and now at University of Miami’s Sylvester Comprehensive Cancer Center.
Mitochondria are essential components of normal cells, so-called “powerhouses” that also play a major role in signaling processes like cell death. There are thousands of mitochondria in each cell. Mitochondria transfer between cells is part of an emerging type of cell-to-cell interaction that is still being explained.
Mitochondria are essential to cancer cells too; chemotherapy and radiation can target mitochondria to destroy tumors. Previous studies established that mitochondria transfer can also happen in other neurological conditions, like stroke, but ongoing research is figuring out the impact of transfer on disease and how it happens.
When cancer cells receive mitochondria, it affects the processes that produce energy. The study found in glioblastoma, this boost supports cancer stem cell properties including self-renewal and tumorigenicity, Dr. Lathia says.
“Cancer – and cancer treatment – does not exist in a vacuum,” Dr. Lathia says. “You’re not just treating and researching the tumors alone, instead tapping into a diverse ecosystem. Further research into this pathway can identify new strategies for treating glioblastoma, but also has potential for understanding other types of cancer.”
Targeting Mitochondria 2023 will extensively cover the implication of mitochondria in cancer and its potential in cancer therapy. Submit a related abstract.
News source: Cleavland Clinic.
Image source: Watson et al. Nature Cancer (2023)
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Iron & Cancer : Targeting Mitochondria
News Release, World Mitochondria Society, Berlin - Germany – May 8, 2023
In their new paper published in Science Advances, Subhadip Mukhopadhyay and colleagues from NYU and Harvard, highlighted a critical link between autophagy, iron metabolism, and mitochondrial function that may have implications for Pancreatic ductal adenocarcinoma (PDAC) progression.
PDAC cells maintain a high level of autophagy, allowing them to thrive in an austere microenvironment. However, the processes through which autophagy promotes PDAC growth and survival are still not fully understood.
Subhadip Mukhopadhyay and his research team showed that autophagy inhibition in PDAC alters mitochondrial function by losing succinate dehydrogenase complex iron sulfur subunit B expression by limiting the availability of the labile iron pool. PDAC uses autophagy to maintain iron homeostasis, while other tumor types assessed require macropinocytosis, with autophagy being dispensable.
The researchers observed that cancer-associated fibroblasts can provide bioavailable iron to PDAC cells, promoting resistance to autophagy ablation. To overcome this cross-talk, they used a low-iron diet and demonstrated that this augmented the response to autophagy inhibition therapy in PDAC-bearing mice.
In summary, this work supports the model in which the iron-autophagy/lysosome axis represents a metabolic vulnerability in PDAC. It specifically demonstrated the feasibility of targeting this metabolic dependency in vivo and mechanistically defined the distinct regulation of iron homeostasis in different tumor types, which has implications for how one would approach this therapeutically.
Targeting Mitochondria 2023 will extensively cover the implication of mitochondria in cancer and its potential in cancer therapy. Submit a related abstract.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Novel drug makes mice skinny even on sugary, fatty diet
News Release, World Mitochondria Society, Berlin - Germany – April 3, 2023
Researchers from The University of Texas Health Science Center at San Antonio (UT Health San Antonio) have developed a small-molecule drug that prevents weight gain and adverse liver changes in mice fed a high-sugar, high-fat Western diet throughout life.
"When we give this drug to the mice for a short time, they start losing weight. They all become slim," said Madesh Muniswamy, PhD, professor of medicine in the health science center's Joe R. and Teresa Lozano Long School of Medicine.
Findings by the collaborators, also from the University of Pennsylvania and Cornell University, were published Feb. 27 in the high-impact journal Cell Reports. Muniswamy, director of the Center for Mitochondrial Medicine at UT Health San Antonio, is the senior author.
Fourth most common element
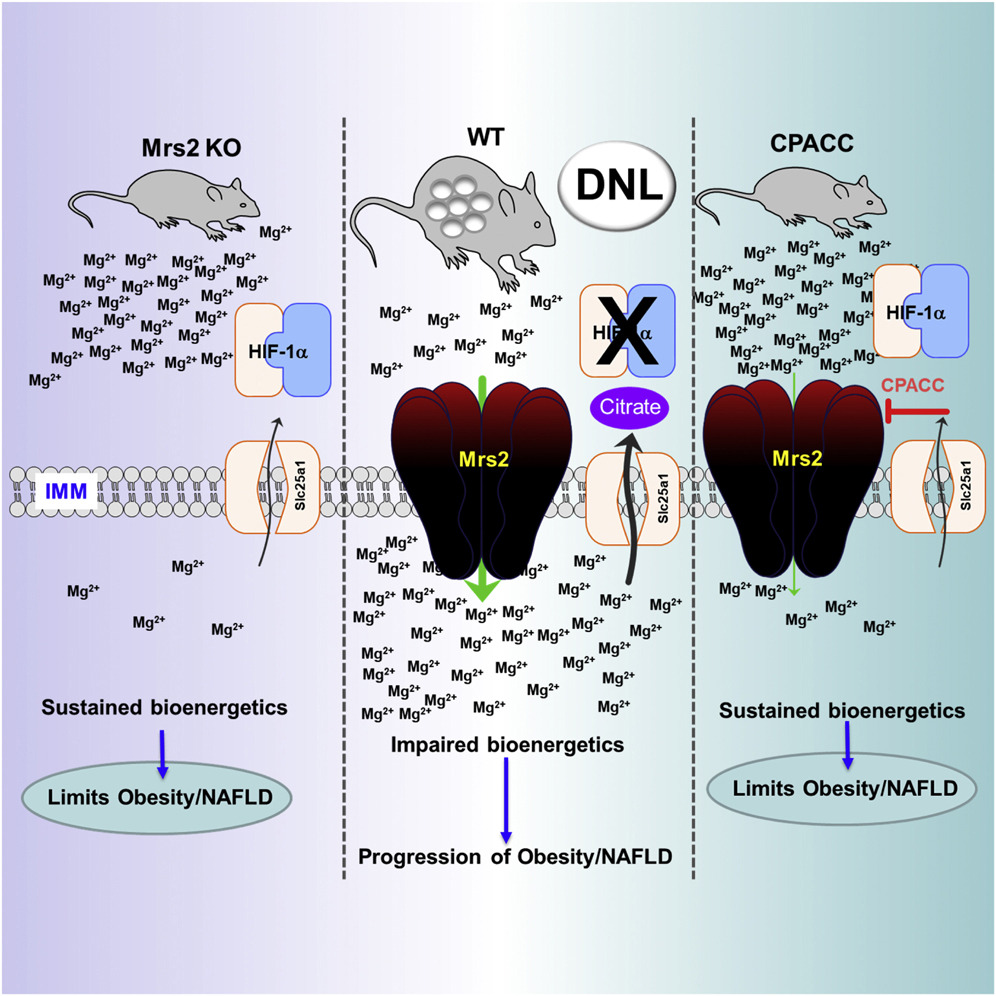
The research team discovered the drug by first exploring how magnesium impacts metabolism, which is the production and consumption of energy in cells. This energy, called ATP, fuels the body's processes.
Magnesium is the fourth most abundant element in the body after calcium, potassium and sodium, and plays many key roles in good health, including regulating blood sugar and blood pressure and building bones. But the researchers found that too much magnesium slows energy production in mitochondria, which are cells' power plants.
"It puts the brake on, it just slows down," said co-lead author Travis R. Madaris, doctoral student in the Muniswamy laboratory at UT Health San Antonio.
Deleting MRS2, a gene that promotes magnesium transport into the mitochondria, resulted in more efficient metabolism of sugar and fat in the power plants. The result: skinny, healthy mice.
Liver and adipose (fat) tissues in the rodents showed no evidence of fatty liver disease, a complication related to poor diet, obesity and type 2 diabetes.
Small-molecule agent
The drug, which the researchers call CPACC, accomplishes the same thing. It restricts the amount of magnesium transfer into the power plants. In experiments, the result was again: skinny, healthy mice. UT Health San Antonio has filed a patent application on the drug.
The mice served as a model system of long-term dietary stress precipitated by the calorie-rich, sugary and fatty Western diet. The familiar results of this stress are obesity, type 2 diabetes and cardiovascular complications.
"Lowering the mitochondrial magnesium mitigated the adverse effects of prolonged dietary stress," said co-lead author Manigandan Venkatesan, PhD, postdoctoral fellow in the Muniswamy lab.
Joseph A. Baur, PhD, of the University of Pennsylvania and Justin J. Wilson, PhD, of Cornell are among the collaborators. "We came up with the small molecule and Justin synthesized it," Madaris said.
Significant implications
"These findings are the result of several years of work," Muniswamy said. "A drug that can reduce the risk of cardiometabolic diseases such as heart attack and stroke, and also reduce the incidence of liver cancer, which can follow fatty liver disease, will make a huge impact. We will continue its development."
Funders of this project include the National Institutes of Health, the U.S. Department of Defense and the San Antonio Partnership for Precision Therapeutics.
Image Credit: Madaris, Travis R. et al. Cell Reports (2023)
Targeting Mitochondria 2023 Conference will elaborate on the latest mitochondria targeting drugs. You can submit your related abstracts here.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Rescuing Corneal Cells from Death with the Help of Mitochondria

News Release, World Mitochondria Society, Berlin - Germany – April 20, 2023
Fuchs' endothelial corneal dystrophy, a degenerative eye disease, causes progressive vision loss that can induce blindness. It is the leading cause of corneal transplantation, but the scarcity of grafts hinders its treatment. A research team from Université Laval and Université de Montréal has identified a way to slow the disease and even avoid transplantation if diagnosed at an early stage.
In people with the disease, the endothelial cells at the back of the cornea die more quickly than in healthy people.
"Everyone loses them at a slow rate, slow enough to make it to the end of our lives without problems. For sick people, depletion is accelerated by factors not yet understood at the molecular level. Since the cells do not divide, they do not replace themselves," says Patrick J. Rochette, full professor at the Faculty of Medicine at Université Laval and a researcher at the CHU de Québec-Université Laval Research Center, who conducted the study.
These endothelial cells play an essential role in vision. They ensure that the cornea remains transparent by keeping it partially dehydrated. When the cells die, the cornea becomes wet and cloudy, which can lead to complete blindness.
In a previous study, the research team showed that mitochondria were central to the disease. "In people with the disease, the mitochondria become depleted rapidly, leading to cell death. The more cells die, the more the mitochondria in other cells have to compensate, which accelerates their depletion. It's a vicious circle," explains Patrick J. Rochette.
Reduce mortality rate
The research team wondered whether injecting healthy mitochondria into cells could delay the progression of Fuchs' dystrophy. To test their hypothesis, the scientists used diseased endothelium removed during a corneal transplant. "We were able to save cells close to death, going from a 60% mortality rate to 10%," Rochette said. These results demonstrate a high therapeutic potential for the injection of mitochondria.
The strength of this approach lies in the automatic recycling of diseased mitochondria without injecting healthy ones directly into the cell. "Cells eat mitochondria as if their life depended on them. Any cell, even if it is dying, will take them up. The replacement happens by itself. After 24 hours, only healthy mitochondria remain," says the researcher.
In the study, the healthy mitochondria were grown under controlled conditions, but the research group is developing an approach to extract them from the patient's blood.
If Fuchs' dystrophy is diagnosed at an early stage, when most endothelial cells are still alive, the approach could maintain vision without a transplant. The injection of mitochondria would be a benign procedure, much less invasive than surgery.
The paper is published in the journal Scientific Reports.
Targeting Mitochondria 2023 will adress the latest research that targets the mitochondria in ophthalmology. You can submit a related abstract.
Source: Press release by the University of Laval.
Image Credits: Freepik
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
Key Information About The Function of Mitochondria in Cancer Cells
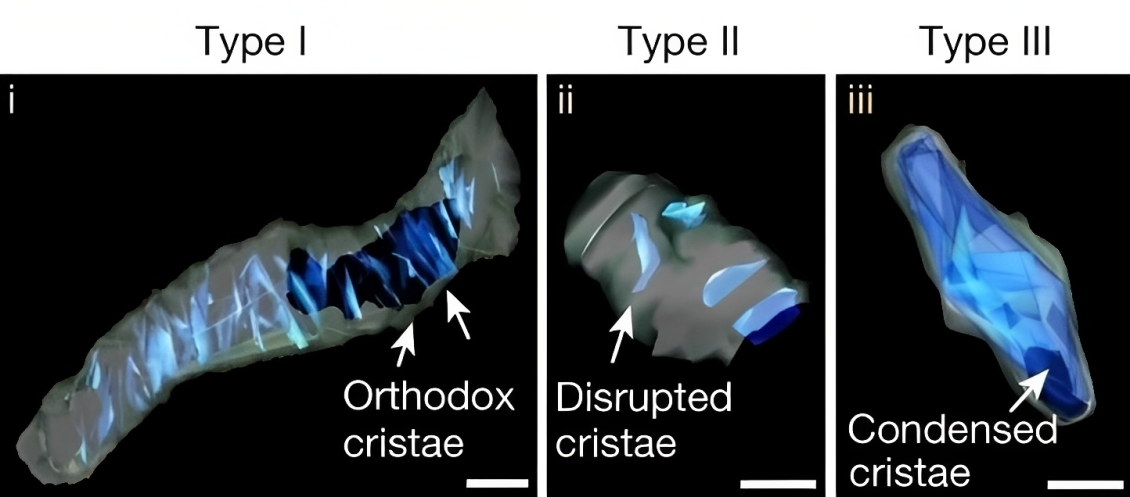
SBEM images and 3D reconstruction of representative type I (i), II (ii) and III (iii) crista structures identified in OXPHOSHI LUAD cells and OXPHOSLO LUSC cells.
News Release, World Mitochondria Society, Berlin - Germany – March 16, 2023
Scientists have long known that mitochondria play a crucial role in the metabolism and energy production of cancer cells. However, until now, little was known about the relationship between the structural organization of mitochondrial networks and their functional bioenergetic activity at the level of whole tumors.
In a new study, published in Nature, researchers from the UCLA Jonsson Comprehensive Cancer Center used positron emission tomography (PET) in combination with electron microscopy to generate 3-dimensional ultra-resolution maps of mitochondrial networks in lung tumors of genetically engineered mice.
They categorized the tumors based on mitochondrial activity and other factors using an artificial intelligence technique called deep learning, quantifying the mitochondrial architecture across hundreds of cells and thousands of mitochondria throughout the tumor.
The authors examined two main subtypes of non-small cell lung cancer (NSCLC)—adenocarcinomas and squamous-cell carcinomas and found distinct subpopulations of mitochondrial networks within these tumors. Importantly, they discovered that the mitochondria frequently organize themselves with organelles such as lipid droplets to create unique subcellular structures that support tumor cell metabolism and mitochondrial activity.
The study was led by Mingqi Han, Ph.D., a post-doctoral researcher in the lab of David Shackelford, Ph.D. Dr. Shackelford is a UCLA Jonsson Comprehensive Cancer Center member and Associate Professor of Pulmonary and Critical Care Medicine at the UCLA David Geffen School of Medicine.
The authors anticipate that mitochondrial populations in human cancer samples will not be mutually exclusive to their respective tumor subtype, but rather there will be a spectrum of activity.
The investigators say these findings provide key information about the function of mitochondria in cancer cells and could lead to new approaches to cancer treatment.
"Our study represents a first step towards generating highly detailed 3-dimensional maps of lung tumors using genetically engineered mouse models," said Dr. Shackelford.
"Using these maps, we have begun to create a structural and functional atlas of lung tumors, which has provided us valuable insight into how tumor cells structurally organize their cellular architecture in response to the high metabolic demands of tumor growth. Our findings hold promise to inform and improve current treatment strategies while illuminating new directions from which to target lung cancer."
"Our study has uncovered a novel finding in the metabolic flux of lung tumors, revealing that their nutrient preference may be determined by the compartmentalization of their mitochondria with other organelles, either relying on glucose ('sugar') or free fatty acids ('fat')," said Dr. Han.
"This discovery has important implications for developing effective anti-cancer therapies that target tumor-specific nutrient preferences. Our multi-modality imaging approach has enabled us to uncover this previously unknown aspect of cancer metabolism, and we believe that it can be applied to other types of cancer, paving the way for further research in this area."
Image Credit: David Shackelford et al, Nature (2023)
Targeting Mitochondria 2023 Conference will elaborate on the latest mitochondria and cancer research and discoveries. You can submit your related abstracts here.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com




























































