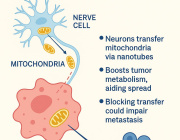
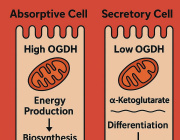
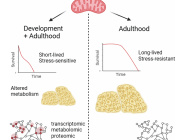
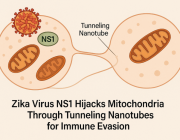
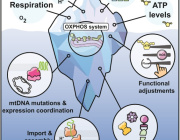
Researchers discover therapeutic target to aid in glaucoma treatment
News Release, World Mitochondria Society, Berlin - Germany – March 13, 2023
Indiana University School of Medicine researchers have identified a new therapeutic target that could lead to more effective treatment of glaucoma.
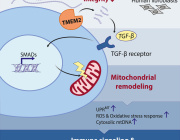
Glaucoma is a neurodegenerative disease that causes vision loss and blindness due to a damaged optic nerve. More than 200,000 people are affected by glaucoma in the United States each year. Unfortunately, there is currently no treatment. In a newly published paper in Communications Biology, researchers found neurons use mitochondria for a steady source of energy, and restoring mitochondrial homeostasis in the diseased neurons can protect the optic nerve cells from being damaged.
“Age-related neurodegenerative disease, which includes glaucoma, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS), is the biggest global health problem,” said Arupratan Das, PhD, assistant professor of ophthalmology and principal investigator of the study. “The fundamental mechanisms that we discovered can be used to protect neurons in glaucoma and be tested for the other diseases. We have identified a critical step of complex mitochondrial homeostasis process, which rejuvenates the dying neuron, similar to giving a lifeline to a dying person.”
The research team, led by Michelle Surma and Kavitha Anbarasu from the Department of Ophthalmology, used induced pluripotent stem cells (iPSCs) from patients with and without glaucoma as well as clustered regularly interspaced short palindromic repeats (CRISPR) engineered human embryonic stem cells with glaucoma mutation. Using stem cell differentiated retinal ganglion cells (hRGCs) of the optic nerve, electron microscopy and metabolic analysis, researchers identified glaucomatous retinal ganglion cells suffer mitochondrial deficiency with more metabolic burden on each mitochondrion. This leads to mitochondrial damage and degeneration. Mitochondria are the tube like structures in cells which produce adenosine triphosphate, cell’s energy source.
However, the process could be reversed by enhancing mitochondrial biogenesis by a pharmacological agent. The team showed retinal ganglion cells are highly efficient in degrading bad mitochondria, but at the same time producing more to maintain homeostasis.
“Finding that retinal ganglion cells with glaucoma produce more adenosine triphosphate even with less mitochondria was astonishing,” Das said. “However, when triggered to produce more mitochondria, the adenosine triphosphate production load was distributed among more mitochondrion which restored the organelle physiology. It is similar to a situation where a heavy stone is carried by fewer people versus a greater number of people—each person will have less pain and injury, just like each mitochondrion will have less difficulty and damage.”
In the future, Das would like to test if these mechanisms protect the optic nerve in animal models under injury before testing in humans to hopefully lead to new clinical interventions.
Targeting Mitochondria 2023 Conference will elaborate on the latest mitochondria research and discoveries. You can submit your related abstracts here.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
An AI-guided screen identifies probucol as an enhancer of mitophagy through modulation of lipid droplets
 Drosophila that represents one of the models of neurodegeneration used in the lab to screen for things (both chemically and genetically) that regulate mitophagy. Credit: Angus McQuibban (CC-BY 4.0)
Drosophila that represents one of the models of neurodegeneration used in the lab to screen for things (both chemically and genetically) that regulate mitophagy. Credit: Angus McQuibban (CC-BY 4.0) News Release, World Mitochondria Society, Berlin - Germany – March 6, 2023
AI analyzes the descriptions of compounds to identify potential new drug candidates.
A new study, published in the journal PLOS Biology, suggests that the language used by researchers in describing their results can be utilized to uncover new treatments for Parkinson’s disease. The study, led by Angus McQuibban of the University of Toronto in Canada, utilized AI to find an existing anti-cholesterol medication that has the capability to enhance the disposal of mitochondria, which are cellular components responsible for energy production and are affected in Parkinson’s disease.
The full pathogenic pathway leading to Parkinson’s disease (PD) is unknown, but one clear contributor is mitochondrial dysfunction and the inability to dispose of defective mitochondria, a process called mitophagy. At least five genes implicated in PD are linked to impaired mitophagy, either directly or indirectly, and so the authors sought compounds that could enhance the mitophagy process.
Several such compounds have been identified, but most of them also cause harm to cells, ruling them out as drug candidates. That led the authors to ask whether the literature describing these compounds might lead them to other compounds, ones not previously linked to mitophagy enhancement but which are described with terms that also appear in papers that discuss the known enhancers.
Identifying patterns of such “semantic similarity” is one of the core skills of IBM Watson for Drug Discovery, an AI program run on a supercomputer that analyzes the published literature for patterns of keywords, phrases, and juxtapositions. The team used the program to develop a semantic “fingerprint” of bona fide mitophagy enhancers, and then looked for similar fingerprints in the literature on a set of over three thousand candidates from a drug database.
The top 79 candidates were screened in cell culture against a mitochondrial poison. The three top candidates from that assay were then tested on several other mitophagy assays, which identified probucol, a cholesterol-lowering drug, as the compound with the best combination of effectiveness and likely safety. Probucol was also found to improve motor function, survival, and neuron loss in two different animal models of Parkinson’s disease (PD is primarily a movement disorder).
Probucol’s effect on mitophagy required the formation and action of lipid droplets, transient cell structures that help maintain mitochondrial integrity during stress, and that accumulate abnormally in Parkinson’s disease. Probucol is known to target ABCA1, a protein involved in lipid transport, and reduction in levels of ABCA1 reduced probucol’s ability to promote mitophagy, suggesting that ABCA1 is a likely mediator of the role of lipid droplets in mitophagy.
“Our study showcased a dual in silico/cell-based screening methodology that identified known and new mechanisms leading to mitophagy enhancement,” McQuibban said. “Given the linkage between lipid droplet accumulation and ABCA1, it seems likely that probucol enhances mitophagy through mobilization of lipid droplets. Targeting this mechanism may be advantageous.”
McQuibban adds, “In our study, we used the AI platform IBM Watson to efficiently identify currently approved drugs that could potentially be re-purposed as therapies for Parkinson’s disease.”
Targeting Mitochondria 2023 Conference will elaborate on the latest mitochondria innovations. You can submit your related abstracts here.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
10 Years on Ferroptosis Discovery: Potential in Disease Treatment
News Release, World Mitochondria Society, Berlin - Germany – February 20, 2023
A new journal article by Columbia professor Brent R. Stockwell marks the ten-year anniversary of the discovery of ferroptosis, a form of cell death that could help treat life-threatening illnesses like cancer.
The average person’s body replaces around 1% of its cells – roughly 330 billion of them – per day. Some cells, like the webbing between fetuses’ fingers and toes, which grows and then disappears before birth, die as a normal part of development. Other cells die of something much like old age; they’re simply programmed to turn out the lights once they’ve done their job. Far scarier than cell death is when cells that should die, don’t, and instead accumulate, causing problems like autoimmune conditions and cancer.
Ten years ago, a team of researchers at Columbia led by Professor Brent R. Stockwell announced a new discovery: A novel kind of cell death that they named “ferroptosis.” When cells undergo ferroptosis, their inner and outer membranes degrade, springing leaks that eventually cause the cell to die. A decade after that initial discovery, Professor Stockwell has released a journal article in Cell this month that assesses what researchers have discovered about ferroptosis in the last 10 years, and how their findings may help treat life-threatening illnesses like cancer and neurodegenerative disease.
“In the ten years since my lab identified its existence, ferroptosis has offered crucial new information on how cells function, and providing insights on how it can help treat disease,” Stockwell said. “We’re confident that over the next decade, ferroptosis will teach us even more.”
Scientists generally recognized three “classic” forms of cell death: Apoptosis, autophagic cell death, and necrosis. Apoptosis occurs naturally in various contexts, such as during fetal development and when cells program themselves to turn off once they’ve aged. In autophagy, damaged cells self-destruct, allowing healthier cells to incorporate their remaining viable material. Necrosis occurs when cells experience trauma, like being cut off from blood supply or infected by disease.
Each of these forms of cell death follows a pattern: In the case of apoptosis, for example, the cell and its nucleus shrink as the cell’s outer layer forms blisters known as blebs. Then, the nucleus collapses as the blisters continue to form. Eventually, the entire cell ruptures, breaking into smaller pieces that float through the bloodstream until they’re swept up by the body’s clean-up cells.
In 2001, while working in his lab, Professor Stockwell started to notice something new: In certain tumor cells treated with a novel chemical he identified, the cells were dying differently and in a manner that depended on their store of iron. Rather than following the patterns observed in apoptosis, these cells were being destroyed primarily because the lipids that form their outer membrane were disintegrating, as he later discovered. The well-known patterns of apoptosis (blebbing, a shrinking nucleus, and so on) didn’t apply. He later ruled out other known forms of cell death as well.
“When I first saw this in my lab, I couldn’t believe it; I had never seen cells die this way, and we knew the implications for the world would be enormous,” Stockwell said. It took 11 years from his initial observation in 2001 until he and his labmembers published their results in 2012, and named the new cell death ferroptosis.
Since 2012, Stockwell and other researchers around the world have conducted numerous studies to understand more about ferroptosis and how it works. They’ve demonstrated that ferroptosis occurs naturally across a range of species, helping execute normal processes that keep organisms running smoothly, like suppressing tumors, and regulating aging.
Researchers have discovered that ferroptosis also occurs in harmful contexts, killing healthy cells in patients with diseases like Parkinson’s, Alzheimer’s and Lou Gehrig’s disease. Ferroptosis can also be triggered by invasive pathogens: A 2021 study indicates that COVID-19 may activate ferroptosis, killing healthy cells during infection.
Though ferroptosis can be destructive, recent studies indicate that it could also be harnessed for good, and may eventually prove a vital tool for fighting disease. Both cancer and autoimmune conditions occur when cells that should die fail to do so. Intentionally inducing ferroptosis could counteract that process, killing cells that should die naturally.
Recent research into links between cancer and ferroptosis has proved particularly fruitful. Several studies conducted over the last several years have shown that deliberately inducing ferroptosis may help stop rampant cancer cell growth in a range of tissues, including lung and pancreas tissue. As scientists learn more, they’ll understand how to best harness ferroptosis to kill dangerous tumor cells in a targeted way.
“We’re so committed to this research because we can see its vast potential,” Stockwell said. “Over the next decade, we hope to unlock all of the life-saving possibilities we can from this discovery. This is a whole new area of of biology that we have unlocked, and we think it will continue to transform biology and medicine.”
Image Credit: Illustration by Nicoletta Barolini
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
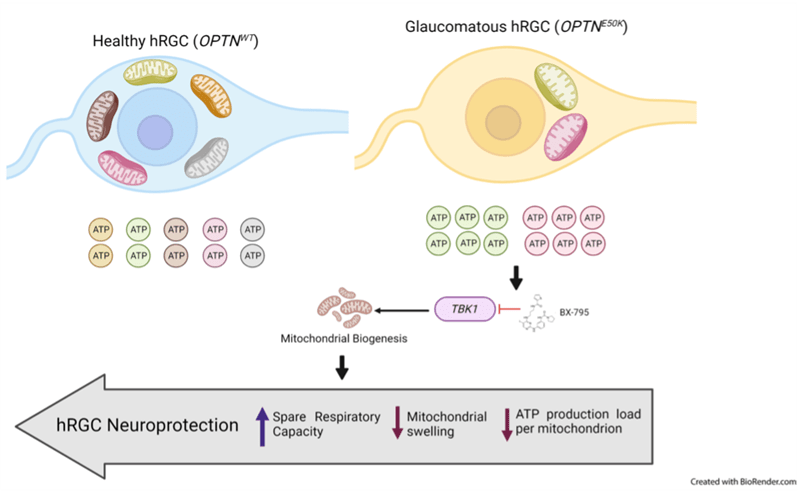
Enhanced Mitochondrial Biogenesis Promotes Neuroprotection
News Release, World Mitochondria Society, Berlin - Germany – March 3, 2023
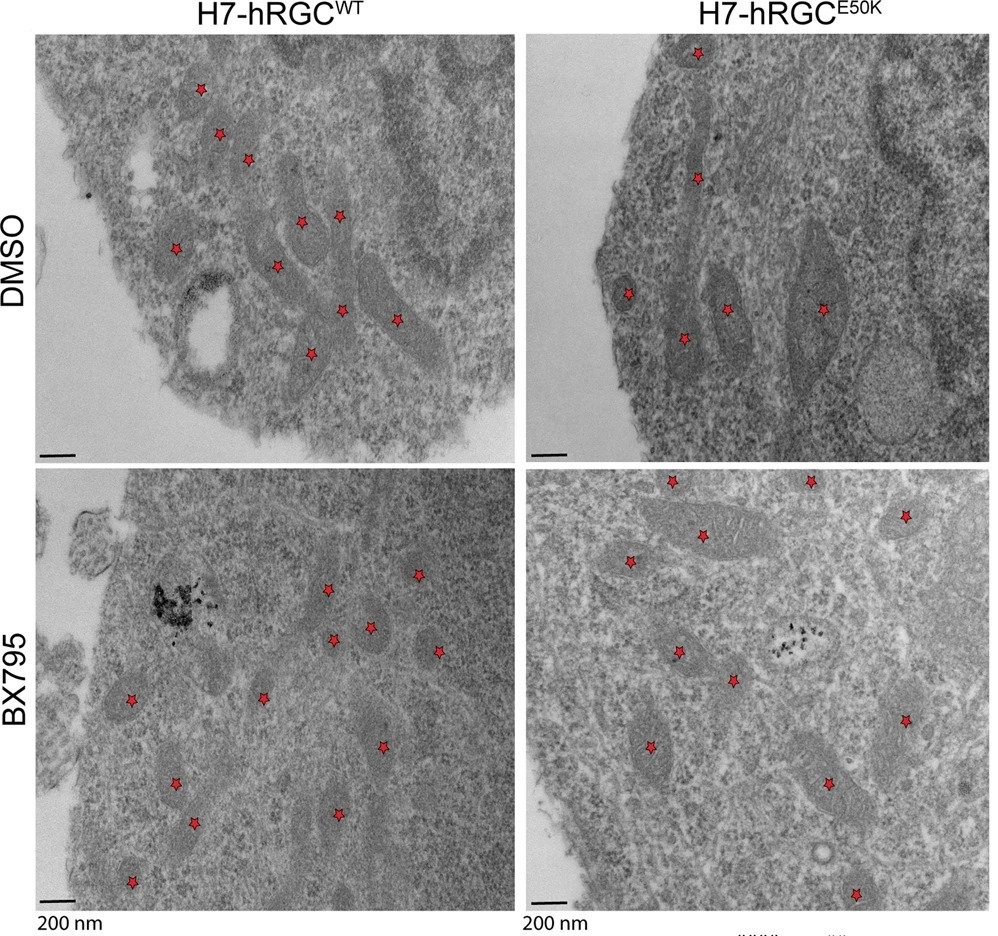
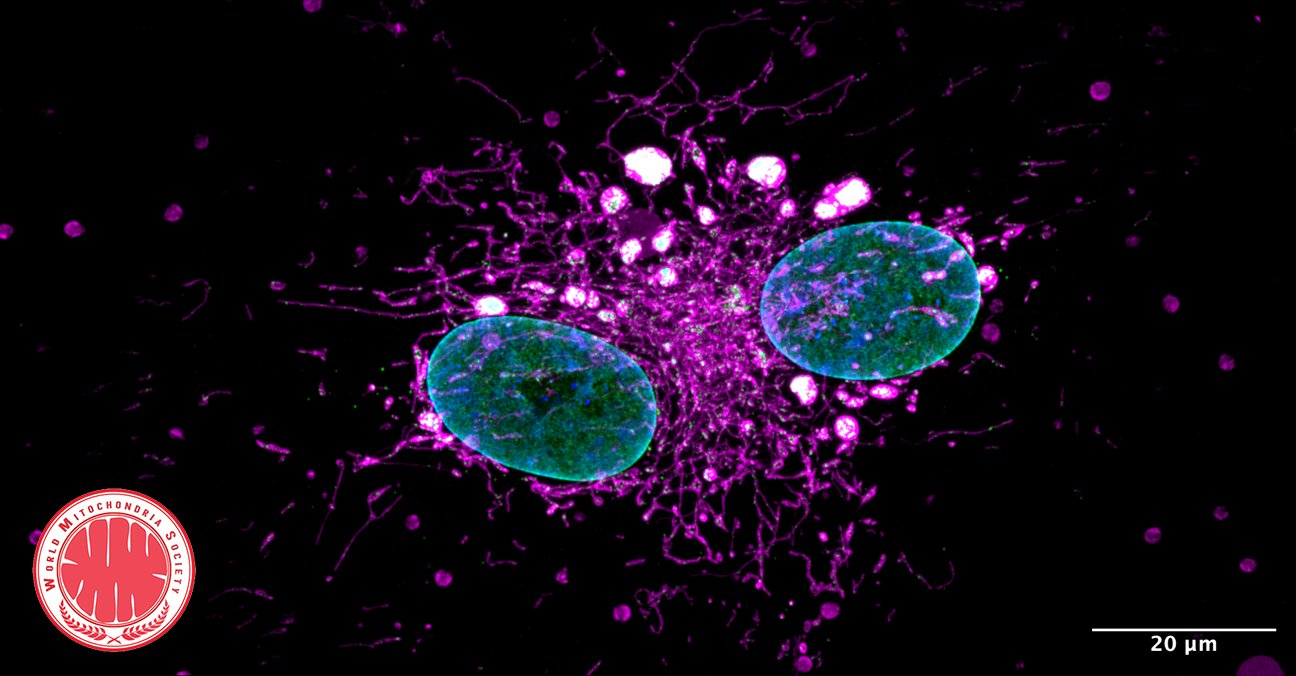
Transmission electron microscopy images of H7-hRGCWT and H7-hRGCE50K after 24 h DMSO or BX795 (1 μg/ml) treatment, at 49000X magnification. Asterisks represent mitochondria.
Mitochondrial dysfunctions are widely afflicted in central nervous system (CNS) disorders with minimal understanding on how to improve mitochondrial homeostasis to promote neuroprotection.
Surma et al. have used human stem cell differentiated retinal ganglion cells (hRGCs) of the CNS, which are highly sensitive towards mitochondrial dysfunctions due to their unique structure and function, to identify mechanisms for improving mitochondrial quality control (MQC).
They showed that hRGCs are efficient in maintaining mitochondrial homeostasis through rapid degradation and biogenesis of mitochondria under acute damage.
Using a glaucomatous Optineurin mutant (E50K) stem cell line, they showed that at basal level mutant hRGCs possess less mitochondrial mass and suffer mitochondrial swelling due to excess ATP production load.
Activation of mitochondrial biogenesis through pharmacological inhibition of the Tank binding kinase 1 (TBK1) restores energy homeostasis, mitigates mitochondrial swelling with neuroprotection against acute mitochondrial damage for glaucomatous E50K hRGCs, revealing a novel neuroprotection mechanism.
Image credit: © Surma et al. Commun Biol (2023).
Targeting Mitochondria 2023 Conference will elaborate on the latest research concerning mitochondria and the nervous system. You can submit your related abstracts here.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com
The secret of slow human brain growth
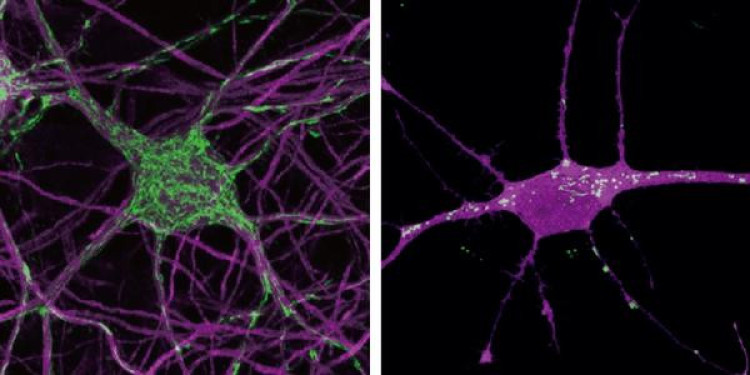
Mouse neurons (left) and a human neuron (right). Mitochondria displayed in green.
News Release, World Mitochondria Society, Berlin - Germany – January 30, 2023
It takes several years for the human brain to develop fully, whereas this happens much faster in other species. The slow maturation of the human brain is thought to be important for its function, but it was unknown what caused this. Now, a team of researchers led by Ryohei Iwata, Pierre Casimir, and Pierre Vanderhaeghen (VIB-KU Leuven Center for Brain & Disease Research and ULB) found that the mitochondria, the energy factory in the brain cells, are responsible for the rate at which the brain develops. This discovery sheds light on human evolution and may have important implications for brain function and diseases. Their work was published in Science.
Pierre Vanderhaeghen: “We discovered that mitochondria set the tempo of neuronal maturation. Neurons have an hourglass inside to measure time, and mitochondria provide that hourglass. This revelation is an important step to understand one of the greatest mysteries in biology: what makes the human brain so distinct from other species, and why is it so sensitive to some diseases? Our findings can also be used to speed up basic and pharmaceutical research into human neurological or psychiatric diseases, which up until now was greatly hindered by slow human neuronal development.”
Driven by mitochondria
The human brain takes a long time to develop its neurons compared to other species. It takes years to reach full maturity, instead of weeks in the mouse. This slow growth is also thought to be crucial for the enhanced functions of the human brain. Previous studies from the Vanderhaeghen Lab showed that this timing is controlled by cells themselves and not external factors, but it was unknown how.
Mitochondria are responsible for energy production in cells. Our brain lives off the energy that the mitochondria in the brain cells produce. Now, Vanderhaeghen and his lab showed that mitochondria also determine the development rate of the brain. This knowledge can have a significant impact on the study of neurological diseases.
The cellular hourglass
Mitochondria are the master regulators of the metabolism in every cell by converting nutrients like sugar into cellular energy. They were thought to be the same in every cell, but Ryohei Iwata and Pierre Casimir made the surprising observation that mitochondria in young human neurons behave differently from those of mouse neurons at the same age: the human mitochondria grow much more slowly, and their energy metabolism is much less active.
Could this be related to the slow speed of human neuron maturation? To test this, they pharmacologically and genetically manipulated the neurons to enhance mitochondrial function. They saw that this accelerated the pace of neuron development, so neurons became more mature months ahead. Conversely, decreasing mitochondrial function led to slower growth in the mouse neurons. This confirmed their suspicion that mitochondria set the tempo of neuronal maturation.
Ryohei Iwata, postdoctoral researcher and first author of the study: “We made this discovery because we developed a new genetic tool to measure time in developing neurons. We also invented a new method to obtain more mature neurons months ahead, which will be highly valuable for medical and pharmaceutical research.”
Implications for brain diseases
Indeed, when studying neuronal diseases in vitro, the slow development of neurons often hampers research. Mimicking the slow pace of maturation observed in vivo, growing human neurons in the lab takes several months to years to reach maturity. This makes the cells particularly difficult to study experimentally. Now, scientists will be able to accelerate neuronal maturation, allowing them to better study the brain’s function and models of neural diseases.
This work also has potentially important implications for some brain diseases. Some diseases strike mitochondria, and the affected patients often show early brain symptoms, which could be related to the discovery reported here. Conversely, some disorders that affect human brain development could be linked to mitochondria. The Vanderhaeghen team will follow up on these critical questions in the future.
News & Image source.Targeting Mitochondria 2023 will keep you updated with the latest discoveries on the mitochondria. You can now submit your abstracts and share your latest work targeting mitochondria during our conference this October.
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2023 Congress
October 11-13, 2023 - Berlin, Germany
wms-site.com