Nerve Cells Damage Could be Reversed Using Peptide
News Release, Wolrd Mitochondria Society, Berlin - Germany – February 22, 2022
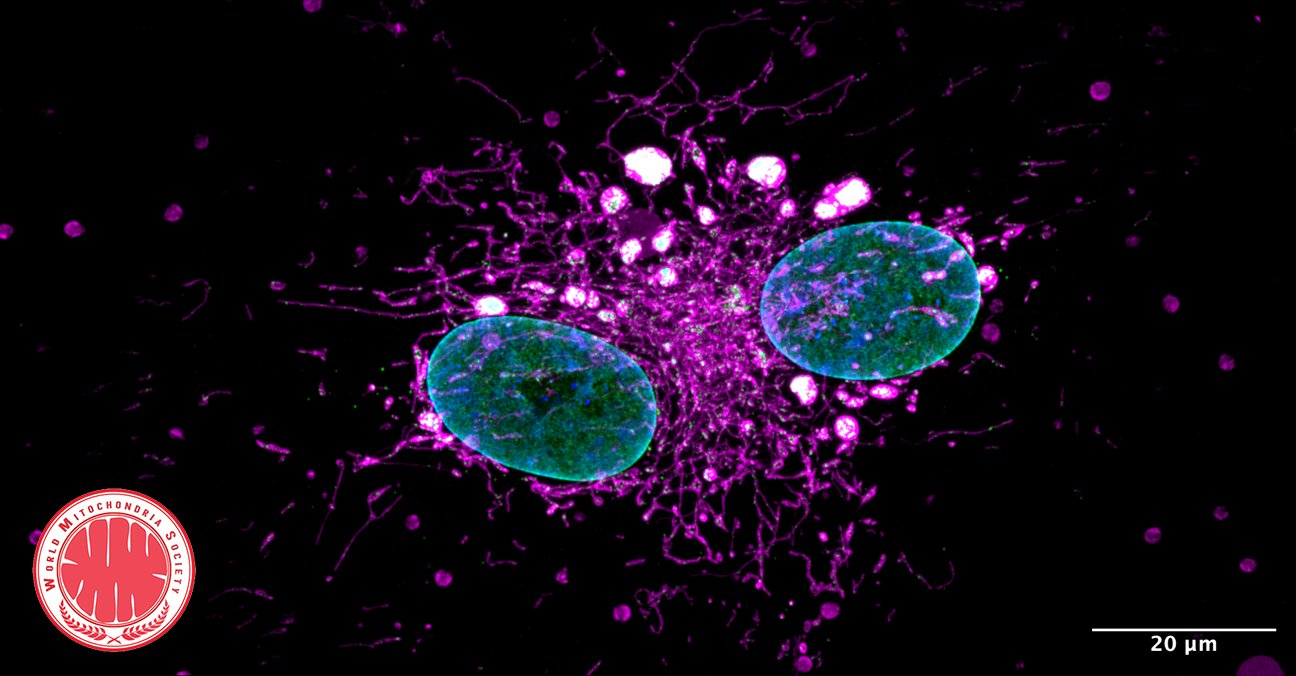
Jun Li et. al research at the University of Illinois Chicago presented promising results for a treatment to stop nerve cell degeneration that happens in some types of disorders, such as hereditary spastic paraplegia and Parkinson's disease, which can cause significant disability.
The reseach team was able to use human cells that they transformed into stem cells and then modified to become nerve cells with the genetic disorder for a particular type of hereditary spastic paraplegia.The study looked at how the long axons that carry messages between nerve cells in the brain can break down, which causes increasingly worse tightening of the leg muscles, leading to imbalance and eventually paralysis, in addition to other symptoms.
Jun Li says: "What we found was that the mitochondria in these cells were breaking apart, what we call mitochondrial fission, and that caused the axons to be shorter and less effective at carrying messages to the brain". Also, "We then looked at whether a particular agent would change the way the nerve cells function - and it did. It inhibited the mitochondrial fission and let the nerve cells grow normally and also stopped further damage."
In conclusion, this agent (a particular chain of amino acids called a peptide) could prove to be useful for a drug or other therapy to stop the nerve cells from becoming damaged or reverse the course of the damage. Moreover, using gene therapy could prevent mitochondrial damage, providing another strategy to reverse the nerve damage.
Similar interesting researches will be discussed in detail in the 13th World Annual Meeting of WMS on Targeting Mitochondria which will be held on October 2022 in Berlin.
Photo Credit: rawpixel.com - www.freepik.com
Media contact:
World Mitochondria Society
This email address is being protected from spambots. You need JavaScript enabled to view it.
+33-1-5504-7755
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
Flavonols Induce Adipose Browning
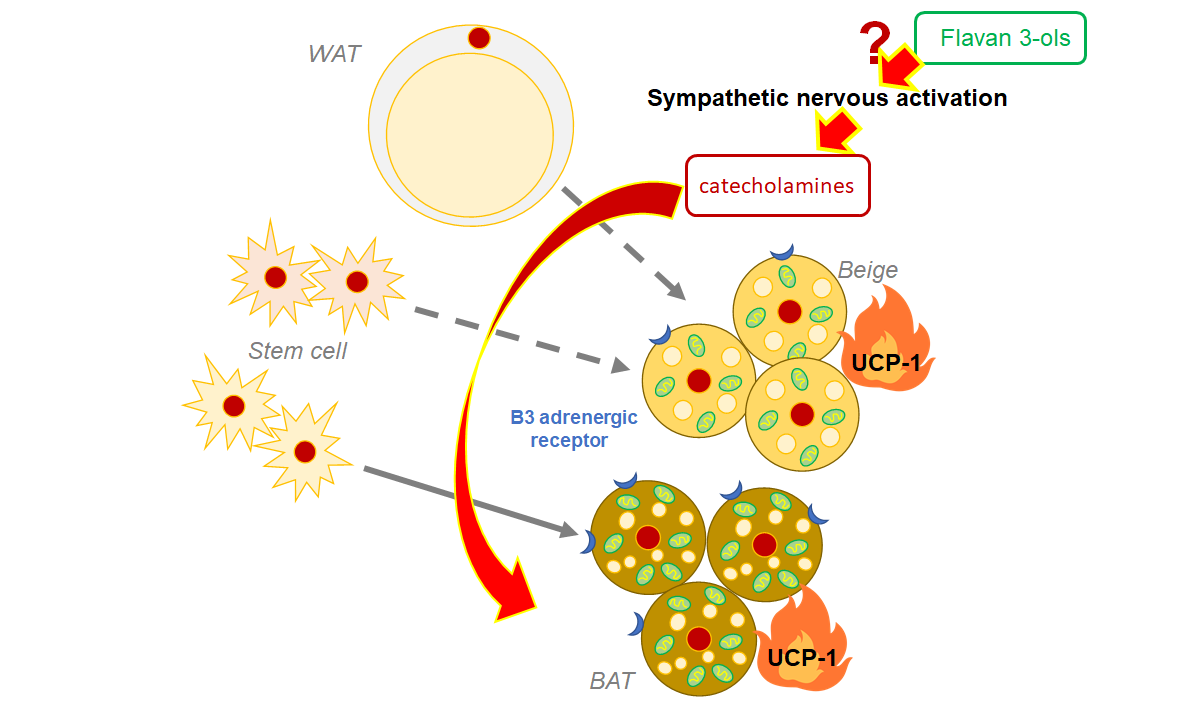
Compared with white adipose tissue, brown adipose tissue BAT has more mitochondria, subcellular organelles associated with energy production, which allows it to burn calories and produce heat by activating the mitochondrial uncoupling protein 1 (Ucp-1).
The stimulation of the sympathetic nervous system (SNS) after cold exposure, exercise, and calorie restriction is well known to induce fat browning. Dietary polyphenols may also activate BAT, causing heat to be dissipated from our bodies.
The authors of this study had previously discovered that a single oral dose of FLs caused fat burning and increased skeletal muscle blood flow. Here, Ishii et al. investigated the effects of single and multiple dose administration of FLs in mouse adipose tissue and found that FLs activate fat browning via the SNS, which secretes "catecholamine" neurotransmitters such as adrenaline (AD) and noradrenaline (NA).
They fed cocoa-derived FLs to distinct groups of mice in two independent sets of experiments. One group was given a single dose of FLs over the course of 24 hours, and their urine was collected for testing. The other group received repeated doses for 14 days before being dissected for the collection of brown and white fat. All adipose samples were tested for gene and protein markers that indicate fat browning, while the urine samples were tested specifically for AD and NA levels.
Higher concentrations of AD and NA in the urine following a single dose of FL clearly demonstrated SNS activation.
The team then used the obtained adipose tissue to investigate the effects of long-term FL treatment. They were thrilled to discover that the white fat of mice who were fed FLs for 14 days eventually turned brown. Some of these cells also had notable structural changes, such as "multilocular phenotype," and appeared to be smaller than normal cells.
Since BAT dissipates heat energy, does long-term FL consumption change the amounts of heat-related proteins? To answer this question, the scientists showed that Ucp-1 levels, as well as other high temperature-linked proteins, increased in mice fed repeated doses of FLs. Browning markers, referred to as "beige markers" in this study, were also abundant in these mice.
"Although the mechanism of adipose browning is not fully understood, it is possible that repeated administration of FLs may produce browning via catecholamines and its receptors," explains Prof. Osakabe. "Further studies will be required to understand how this process is induced by FL-rich foods," she concludes.
Adipose browning will be discussed in detail in the 13th World Annual Meeting of WMS on Targeting Mitochondria will be held on October 2022 in Berlin.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
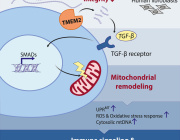
Beyond mitochondria: Alternative energy-producing pathways from all strata of life
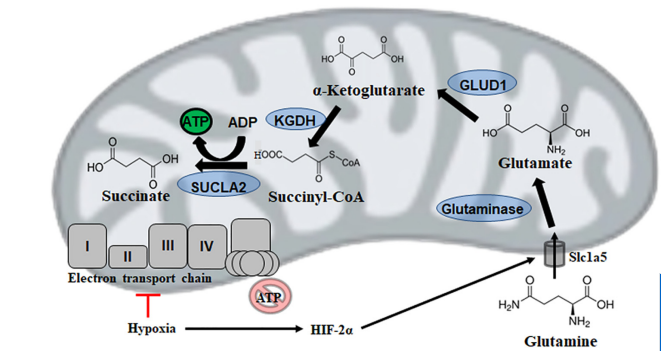
Mitochondrial substrate level phosphorylation via succinate-CoA ligase enables survival despite a defective electron transport chain.
This brilliant study by Christopher Auger et. al reviews alternative energy-producing pathways from all strata of life.
Mitochondria are the powerhouses of the cell for they produce adenosine triphosphate (ATP), the universal energy currency. However, intricacy and efficiency, the most significant strengths of the electron transport chain (ETC), are also its greatest downfalls.
A reliance on metal complexes, lipid moities, and cofactors renders oxidative phosphorylation vulnerable to environmental toxins, intracellular reactive oxygen species (ROS) and fluctuations in diet. Thus, it is of interest to note that temporal disruptions in ETC activity in most organisms are rarely fatal, and often a redundant number of failsafes are in place to permit continued ATP production when needed.
This review highlights the metabolic reconfigurations discovered in organisms ranging from parasitic Entamoeba to bacteria such as pseudomonads and then complex eukaryotic systems that allow these species to adapt to and occasionally thrive in harsh environments.
The aim of this review is to demonstrate the plasticity of metabolic networks and recognize that in times of duress, life finds a way.
The author of this paper, Dr. Marc G. Jeschke, will join us during the 13th World Congress in October to present all his recent results concerning mitochondrial function and associate metabolic changes in a model of severe trauma
Read more about this article: 10.1016/j.metabol.2021.154733
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
www.targeting-mitochondria.com
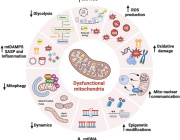
Mitophagy dysfunction in mitochondrial muscle disease
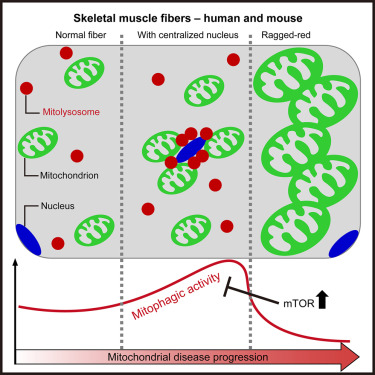
Mitophagy is a quality control mechanism that eliminates damaged mitochondria, yet its significance in mammalian pathophysiology and aging has remained unclear. Here, Mito et al., report that mitophagy contributes to mitochondrial dysfunction in skeletal muscle of aged mice and human patients.
The early disease stage is characterized by muscle fibers with central nuclei, with enhanced mitophagy around these nuclei. However, progressive mitochondrial dysfunction halts mitophagy and disrupts lysosomal homeostasis. Interestingly, activated or halted mitophagy occur in a mosaic manner even in adjacent muscle fibers, indicating cell-autonomous regulation. Rapamycin restores mitochondrial turnover, indicating mTOR-dependence of mitochondrial recycling in advanced disease stage.
Their evidence suggests that:
- Mitophagy is a hallmark of age-related mitochondrial pathology in mammalian muscle.
- Mosaic halting of mitophagy is a mechanism explaining mosaic respiratory chain deficiency and accumulation of pathogenic mtDNA variants in adult-onset mitochondrial diseases and normal aging.
- Augmenting mitophagy is a promising therapeutic approach for muscle mitochondrial dysfunction.
We will be discussing mitochondrial dysfunctions further in the 13th World Congress on Targeting Mitochondria held on October 26-28, 2022 in Berlin.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com
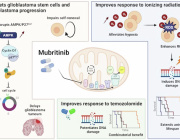
Mitochondrial Transfer in Cardiovascular Disease: From Mechanisms to Therapeutic Implications
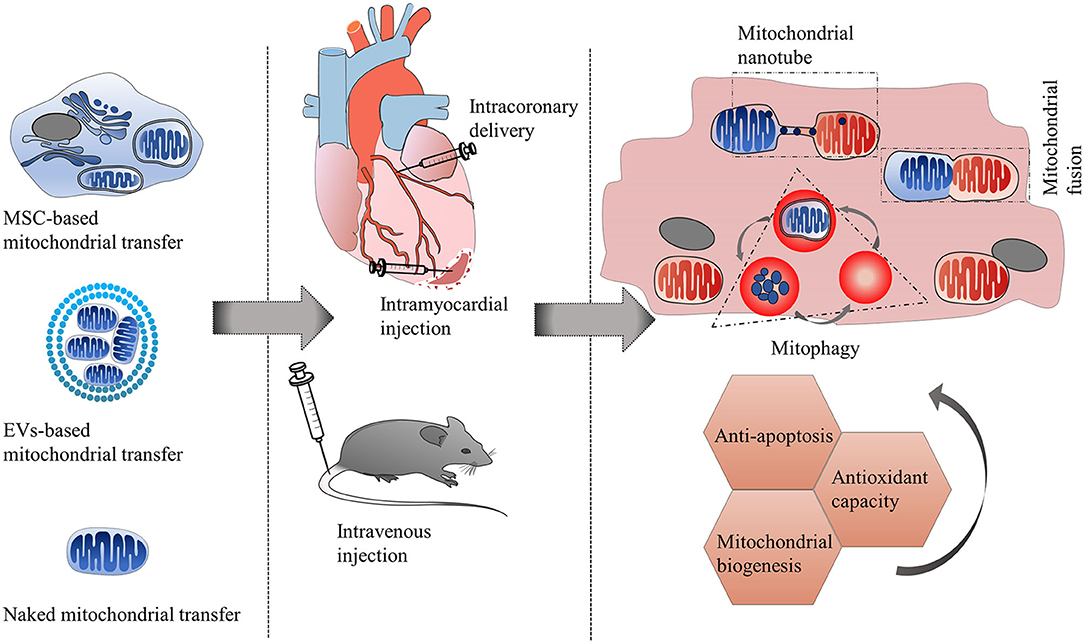
Therapeutic strategies of mitochondrial transfer for cardiovascular diseases.
This informative review by Chen et al. studied the phenomenon of mitochondrial transfer in cardiovascular diseases.
Mitochondrial dysfunction has been proven to play a critical role in the pathogenesis of cardiovascular diseases. The phenomenon of intercellular mitochondrial transfer has been discovered in the cardiovascular system. This cell-to-cell mitochondrial transfer plays an essential role in regulating cardiovascular system development and maintaining normal tissue homeostasis under physiological conditions. In pathological conditions, damaged cells transfer dysfunctional mitochondria toward recipient cells to ask for help and take up exogenous functional mitochondria to alleviate injury.
In this review, they summarized the mechanism of mitochondrial transfer in the cardiovascular system and outlined the fate and functional role of donor mitochondria. They also discussed the advantage and challenges of mitochondrial transfer strategies, including cell-based mitochondrial transplantation, extracellular vesicle-based mitochondrial transplantation, and naked mitochondrial transplantation, for the treatment of cardiovascular disorders.
This topic and many more are to be presented in our 13th Annual Meeting 2022 - in Germany
Authors: Jun Chen, Jinjie Zhong, Lin-lin Wang, and Ying-ying Chen
Read full article.