Mitochondria, general intelligence, health and aging
The Scientific committee would like to share this excellent article written by Eric Stann on Intelligence can link to health and aging.
Credit: University of Missouri
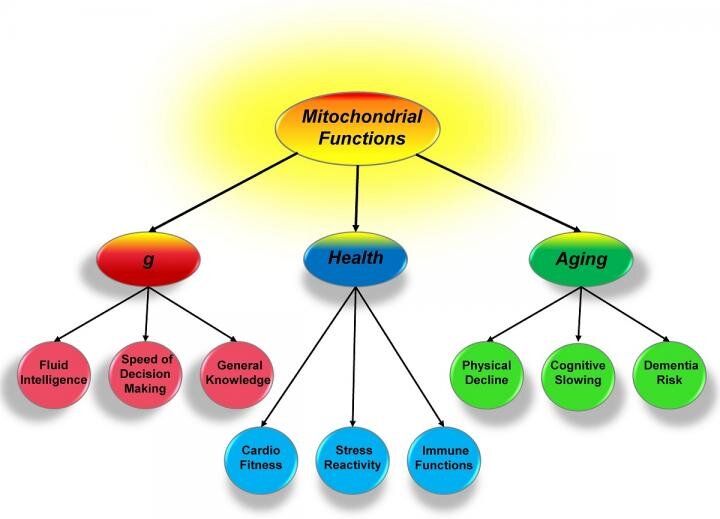
As Introduction: For over 100 years, scientists have sought to understand what links a person's general intelligence, health and aging. In a new study, a University of Missouri scientist suggests a model where mitochondria, or small energy producing parts of cells, could form the basis of this link. This insight could provide valuable information to researchers studying various genetic and environmental influences and alternative therapies for age-related diseases, such as Alzheimer's disease.
To Conclude: These systems are being used over and over again, and eventually their heavy use results in gradual decline. Knowing this, we can help explain the parallel changes in cognition and health associated with aging. Also with good mitochondrial function, the aging processes will occur much more slowly. Mitochondria have been relatively overlooked in the past, but are now considered to relate to psychiatric health and neurological diseases.
Article link: https://www.eurekalert.org/pub_releases/2019-05/uom-icl050819.php
Targeting Mitochondria 2021 Congress
October 27-29, 2021
Berlin, Germany & Virtual Congress
www.targeting-mitochondria.com
Big change from small player -- Mitochondria alter body metabolism and gene expression
The Scientific Committee of the World Mitochondria Society would like to share this article by Scott Ballinger on Big change from small player Mitochondria alter body metabolism and gene expression.
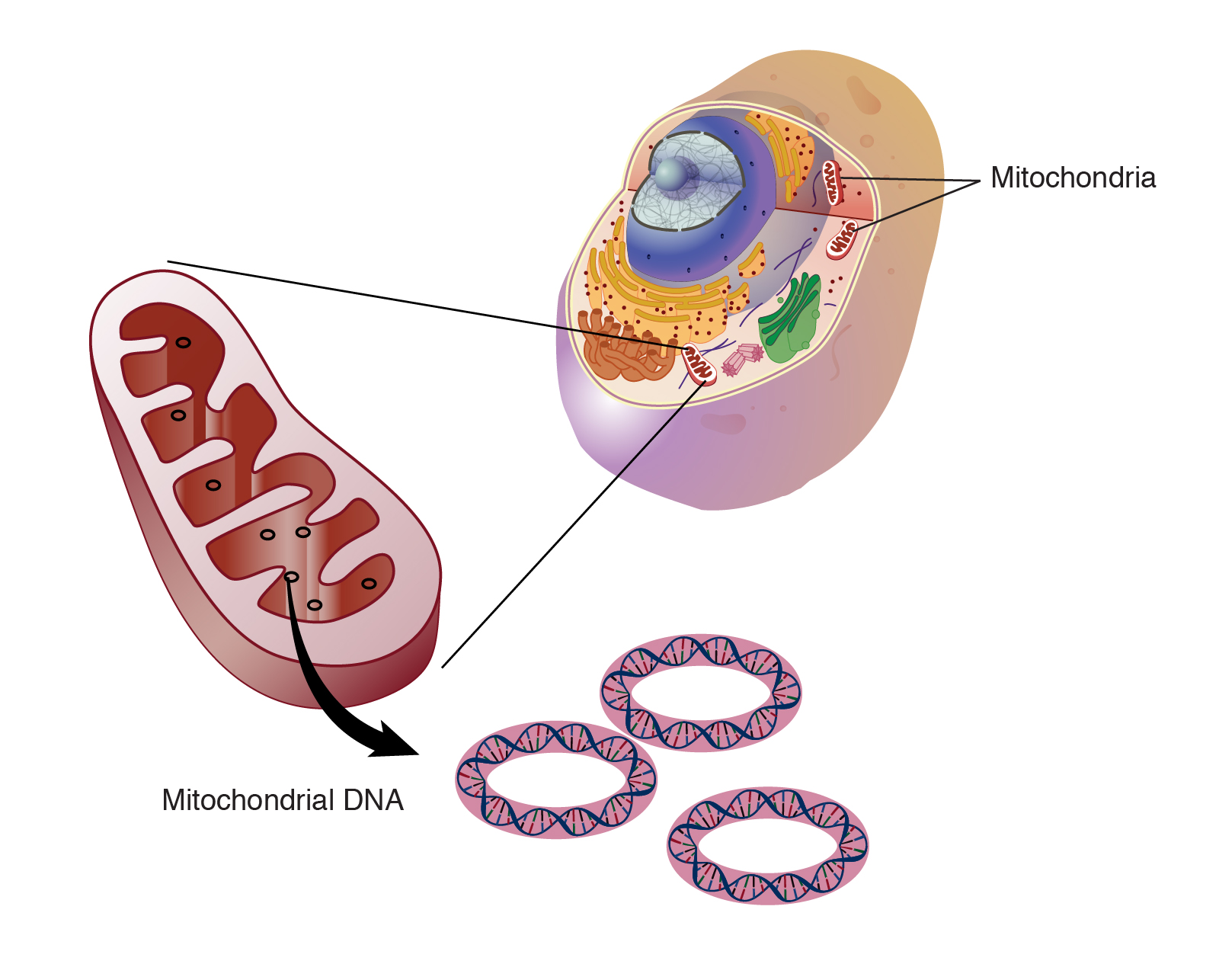
Image: mitochondrial_dna
The Scope is: About 1.5 billion years ago, tiny visitors came to live inside the cells that later evolved into all plant and animal life including humans.Those visitors were mitochondria, small organelles whose prominent role is producing 90 percent of the chemical energy cells need to survive. Evolutionarily speaking, humans, animals and plants are thus a combination of two organisms.Mitochondria have their own DNA, but the 13 genes in human mitochondria — along with DNA sequences for tRNAs, rRNAs and some small peptides — are massively overshadowed by the 20,000 genes in the human nucleus. Nevertheless, these diminutive mitochondria may have a strong influence on cellular metabolism and susceptibility to metabolic diseases like heart failure or obesity.
The Conclusion is: These results are clearly consistent with the notion that different nuclear-mitochondrial genetic combinations influence metabolism, adiposity and gene expression in different ways,” Ballinger said. “The overall implication of this work is that it can provide a new framework for understanding complex genetic disease susceptibility — that both an individual’s nuclear and mitochondrial genomes, in combination, can affect disease development. We are now trying to understand how the different combinations of nuclear and mitochondrial encoded genes interact to alter metabolism, and how this influences individual disease susceptibility.
Full Article: https://www.eurekalert.org/pub_releases/2018-11/uoaa-bcf110918.php
Targeting Mitochondria 2021 Congress
October 27-29, 2021
Berlin, Germany & Virtual Congress
www.targeting-mitochondria.com
Interaction of mitochondria and lysosomes key in Parkinson's disease
The Scientific committee would like to share this excellent article written by Soojin Kim and al. on Interaction of mitochondria and lysosomes key in Parkinson's disease.
Credit: Northwestern University
The Scope is: Mitochondria-lysosome contacts are recently identified sites for mediating crosstalk between both organelles, but their role in normal and diseased human neurons remains unknown. In this study, we demonstrate that mitochondria-lysosome contacts can dynamically form in the soma, axons, and dendrites of human neurons, allowing for their bidirectional crosstalk.
The demonstaration is: That M–L contact sites dynamically form in human neurons, and further investigates their role in neurons from patients with GBA1-linked PD. We found that loss of lysosomal GCase enzymatic activity in PD patient-derived dopaminergic neurons led to prolonged M–L contact tethering dynamics due to defective contact untethering machinery, and resulted in misregulated axonal distribution of mitochondria and decreased ATP levels.
DOI: 10.1038/s41467-021-22113-3
Parkinson, cancer, type 2 diabetes share a key element that drives disease
This study talk about Parkinson's, cancer, type 2 diabetes share a key element that drives disease written by Chien-Min Hung and Al.
Credit: Salk Institute
The Scope is: The serine/threonine kinase ULK1 mediates autophagy initiation in response to various cellular stresses, and genetic deletion of ULK1 leads to accumulation of damaged mitochondria. Here we identify Parkin, the core ubiquitin ligase in mitophagy, and PARK2 gene product mutated in familial Parkinson’s disease, as a ULK1 substrate.
In conclusion: This study has identified Ser108 as a novel site of Parkin regulation directly downstream of AMPK/ULK1 pathway activation and forces a revision of dogma regarding when and where Parkin function may be important. The ability of ULK1 to phosphorylate Ser108 in the Parkin ACT element following even mild mitochondrial stresses—including metformin—begets questions of whether this event serves as an “early alert signal” of mitochondrial damage and may play a surveillance/proteostatic role in some biological contexts by modulating Parkin interactions before full Parkin catalytic activation.
Mitochondrial enzyme found to block cell death pathway points to new cancer treatment strategy
The Scientific Committee of the World Mitochondria Society would like to share this article by Chao Mao and al. on Mitochondrial enzyme found to block cell death pathway points to new cancer treatment strategy.
Credit: CC0 Public Domain
The Scope is: The mitochondrial enzyme dihydroorotate dehydrogenase (DHODH) plays an important and previously unknown role in blocking a form of cell death called ferroptosis, according to a new study by researchers at The University of Texas MD Anderson Cancer Center. Preclinical findings suggest that targeting DHODH can restore ferroptosis-driven cell death, pointing to new therapeutic strategies that may be used to induce ferroptosis and inhibit tumor growth.
To conclude: In GPX4-low cancers, brequinar effectively induced ferroptosis and suppressed tumor growth, but the effects were not seen in GPX4-high cancers. However, the combination of brequinar and sulfasalazine, an FDA-approved ferroptosis inducer, resulted in a synergistic effect to overcome high GPX4 expression and to block tumor growth.
DOI: 10.1038/s41586-021-03539-7




























































