New possibilities to prevent sudden cardiac death
Nearly a half-million people a year die from sudden cardiac death (SCD) in the U.S. -- the result of malfunctions in the heart's electrical system.
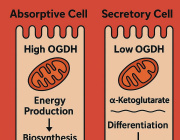
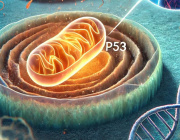
A leading cause of SCD in young athletes is arrhythmogenic cardiomyopathy (ACM), a genetic disease in which healthy heart muscle is replaced over time by scar tissue (fibrosis) and fat.
Stephen Chelko, an assistant professor of biomedical sciences at the Florida State University College of Medicine, has developed a better understanding of the pathological characteristics behind the disease, as well as promising avenues for prevention. His findings are published in the current issue of Science Translational Medicine.
Individuals with ACM possess a mutation causing arrhythmias, which ordinarily are non-fatal if managed and treated properly. However, Chelko shows that exercise not only amplifies those arrhythmias, but causes extensive cell death. Their only option is to avoid taking part in what should be a healthy and worthwhile endeavor: exercise.
"There is some awful irony in that exercise, a known health benefit for the heart, leads to cell death in ACM subjects," Chelko said. "Now, we know that endurance exercise, in particular, leads to large-scale myocyte cell death due to mitochondrial dysfunction in those who suffer from this inherited heart disease."
Several thousand mitochondria are in nearly every cell in the body, processing oxygen and converting food into energy. Considered the powerhouse of all cells (they produce 90 percent of the energy our bodies need to function properly), they also play another important role as a protective antioxidant.
As mitochondria fail to function properly, and myocyte cells in the heart die, healthy muscles are replaced by scar tissue and fatty cells. Eventually, the heart's normal electrical signals are reduced to an erratic and disorganized firing of impulses from the lower chambers, leading to an inability to properly pump blood during heavy exercise. Without immediate medical treatment, death occurs within minutes.
Chelko's research gets to the heart of the process involved in mitochondrial dysfunction.
"Ultimately, mitochondria become overwhelmed and expel 'death signals' that are sent to the nucleus, initiating large-scale DNA fragmentation and cell death," Chelko said. "This novel study unravels a pathogenic role for exercise-induced, mitochondrial-mediated cell death in ACM hearts."
In addition to providing a better understanding of the process involved, Chelko discovered that cell death can be prevented by inhibiting two different mitochondrial proteins. One such approach utilizes a novel targeting peptide developed for Chelko's research by the National Research Council in Padova, Italy.
That discovery opens avenues for the development of new therapeutic options to prevent myocyte cell death, cardiac dysfunction and the pathological progression leading to deadly consequences for people living with ACM.
This research was funded by an American Heart Association Career Development Award.
News source: www.sciencedaily.com
New way to halt excessive inflammation

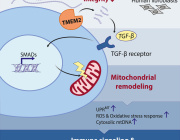
RCSI researchers have discovered a new way to 'put the brakes' on excessive inflammation by regulating a type of white blood cell that is critical for our immune system.
The discovery has the potential to protect the body from unchecked damage caused by inflammatory diseases.
The paper, led by researchers at RCSI University of Medicine and Health Sciences, is published in Nature Communications.
When immune cells (white blood cells) in our body called macrophages are exposed to potent infectious agents, powerful inflammatory proteins known as cytokines are produced to fight the invading infection. However, if these cytokine levels get out of control, significant tissue damage can occur.
The researchers have found that a protein called Arginase-2 works through the energy source of macrophage cells, known as mitochondria, to limit inflammation. Specifically they have shown for the first time that Arginase-2 is critical for decreasing a potent inflammatory cytokine called IL-1.
This discovery could allow researchers to develop new treatments that target the Arginase-2 protein and protect the body from unchecked damage caused by inflammatory diseases.
"Excessive inflammation is a prominent feature of many diseases such as multiple sclerosis, arthritis and inflammatory bowel diseases. Through our discovery, we may be able to develop novel therapeutics for the treatment of inflammatory disease and ultimately improve the quality of life for people with these conditions," commented senior author on the paper Dr Claire McCoy, Senior Lecturer in Immunology at RCSI.
The study was led by researchers at the School of Pharmacy and Biomolecular Sciences, RCSI (Dr Claire McCoy, Dr Jennifer Dowling and Ms Remsha Afzal) in collaboration with a network of international researchers from Australia, Germany, and Switzerland.
The research was funded by Science Foundation Ireland, with initial stages of the research originating from a grant from the National Health Medical Research Council, Australia.
News source: www.sciencedaily.com
Dowling, J.K., Afzal, R., Gearing, L.J. et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat Commun 12, 1460 (2021). https://doi.org/10.1038/s41467-021-21617-2
Mitochondrial dysfunction caused by bacteria activates intrinsic apoptosis and inflammation
Monash Biomedicine Discovery Institute (BDI) scientists have discovered a previously unknown method used by bacteria to evade immune responses.
The study, published in Nature Microbiology, points to potential new ways of countering bacterial infections, which are becoming increasingly resistant to antibiotics.First author Dr Pankaj Deo said researchers in Dr Thomas Naderer’s laboratory took a different approach to understanding the process by which bacteria release toxins that disarm the “powerhouse” mitochondria in immune cells.The study showed that immune cells sense that their mitochondria are no longer functional during infections, which triggers apoptosis. “Ironically, it is the activation of host cell death factors that deliver the final blow to mitochondria which induces apoptosis, not the bacterial toxins themselves,” Dr Pankaj said.The researchers genetically targeted apoptotic factors and showed that they were able to reduce inflammation in mice, which increased health outcomes.They used the bacterial pathogens Neisseria gonorrhoeae, uropathogenic Escherichia coli and the deadly Pseudomonas aeruginosa, prevalent in hospitals and which can be multi-drug resistant. However, the findings would apply to other species of bacteria too, Dr Deo said.Dr Naderer, who oversaw the research, said that understanding the ways some bacterial infections evade immune response by targeting mitochondria opens new therapeutic possibilities.“There’s been a lot of effort trying to block endotoxins that kill immune cells but this study really shifts the focus onto different toxins that might be more important,” Dr Naderer said.“It gives us a few good leads that we can look at as a next step,” he said.“We’ve shown in this paper that we can accelerate the immune response,” he said. “The other side is that if that response persists and we get constant inflammation – which is usually associated with bacterial infection and which causes a lot of tissue damage – we have a new way to shut down that tissue-damaging inflammation.”“What scientists have thought before is that when endotoxins are released by bacteria they induce an inflammatory type of programmed cell death called pyroptosis in immune cells,” Dr Deo said. Endotoxins are part of the external cell wall of essentially all Gram-negative bacteria.“We’ve found that the pathogenic bacteria use a similar mechanism to release additional toxins,” he said. “They kill immune cells by releasing small surface structures called outer membrane vesicles – packages of toxins that target mitochondria. The mitochondria are disarmed, become dysfunctional then die according to apoptosis or cellular suicide.”The scientists will investigate drugs that are now advancing to the clinic, and at repurposing drugs already in use, perhaps as anti-cancer treatments, to see if they can be used to clear bacterial infections.
News source: www.technologynetworks.com
Article: Deo et al. (2020). Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nature Microbiology. DOI: https://doi.org/10.1038/s41564-020-0773-2
Mitochondrial function can play significant part in serious disease

Disorders of the cells' energy supply can cause a number of serious diseases, but also seem to be connected to ageing. More research is needed on mitochondrial function to find future treatments. A new study involving researchers at Karolinska Institutet shows how an important molecule inside the mitochondria affects their function in mice and fruit flies. The study, which is published in Science Advances, adds valuable knowledge on formerly relatively unexplored protein modifications.
In each cell of the body is an organ called the mitochondrion, which converts nutrients in our food to energy. Mitochondria are an essential part of the metabolism, and when things go wrong we can develop serious diseases.
Mitochondrial dysfunction is the hallmark of a group of rare genetic disorders but can also be observed in common diseases such as diabetes, heart disease, neurodegenerative diseases and the normal ageing process.
More research is needed on mitochondria and how they communicate with the rest of the cell if scientists are to find new therapeutic approaches to improve mitochondrial function.
Researchers at Karolinska Institutet, the Max-Planck Institute for Biology of Ageing in Cologne and the University of California San Diego have now studied how the methylation of proteins affects different mitochondrial processes.
Methylation is a chemical modification in which a methyl group (CH3) is added to a molecule, thereby potentially affecting its function. S-Adenosylmethionine (SAM), also known as AdoMet, is the main methyl group donor within the cell, including inside of mitochondria.
"We're interested in studying this particular molecule since the production of SAM changes in cancer and when we age," says Anna Wredenberg, researcher at the Department of Medical Biochemistry and Biophysics, Karolinska Institutet.
By completely removing SAM from the mitochondria of fruit flies and mice, the researchers have been able to study which processes in the mitochondria are dependent on methylation.
"Earlier studies have shown that both SAM and cellular energy levels drop during ageing. Our study suggests a link between these two pathways by demonstrating that low SAM levels can influence mitochondrial energy production."
The study has identified which of the mitochondrial proteins are methylated and how methylation affects them, and how these modifications might affect mitochondrial function. The researchers also demonstrate the physiological consequences of the lack of such changes. However, several questions still need answering.
"Our study has provided an indication that some modifications can be modulated by diet, but we need to continue examining if we can change the pathological process for the better," says Anna Wredenberg. "So far we've only looked at protein changes, but other molecules can also be modified by intra-mitochondrial SAM. We have to study these modifications to get a better understanding of the role it plays."
The study was financed by the Swedish Research Council, the European Research Council, the Knut and Alice Wallenberg Foundation, the Max Planck Society and the Ragnar Söderberg Foundation. There are no reported conflicts of interest.
News Source: https://www.sciencedaily.com/releases/2021/02/210219155928.htm
Florian A. Schober, David Moore, Ilian Atanassov, Marco F. Moedas, Paula Clemente, Ákos Végvári, Najla El Fissi, Roberta Filograna, Anna-Lena Bucher, Yvonne Hinze, Matthew The, Erik Hedman, Ekaterina Chernogubova, Arjana Begzati, Rolf Wibom, Mohit Jain, Roland Nilsson, Lukas Käll, Anna Wedell, Christoph Freyer, Anna Wredenberg. The one-carbon pool controls mitochondrial energy metabolism via complex I and iron-sulfur clusters. Science Advances, 2021; 7 (8): eabf0717 DOI: 10.1126/sciadv.abf0717
Who attended the Targeting Mitochondria 2020?
| Academia Sinica AgResearch, a Crown Research Insitute Ajou University Amsterdam UMC, VUmc Angers University Hospital Center APC Microbiome Augusta University Augusta University/College of Dental Medicine BASF SE Beiersdorf AG Benemérita Universidad Autónoma de Puebla Biochemia Zdrowia Krzysztof Michalak Biomedical Research Foundation of the Academy of Athens Brown University Bucheon St. Mary's hospital Center For Neuroscience And Cell Biology Center of Experimental Medicine SAS Champalimaud Foundation Children's Hospital of Philadelphia Christian Albrechts University Kiel Chubu University CHUV-Unil CiRA, Kyoto University Cleveland Clinic Lerner Research Institute CNC - Center for Neuroscience and Cell Biology College of Health Professions Comenius University in Bratislava Defence Institute of Physiology and Allied Sciences Department of Neurology, Otto-von-Guericke-University, Magdeburg, Germany Dept Toxicogenomics, School for Mental Health and Neuroscience /Maastricht University EA Pharma Co., Ltd. Emory University EPFL Epirium Bio Etablissement Français du Sang Nouvelle Aquitaine ETH Zurich Facultad de Medicina Universidad Autonoma de Nuevo Leon FRC Kazan Scientific Center FRC Kazan Scientific Center of RAS FUNDACIÓN INVESTIGACIÓN BIOMÉDICA HOSPITAL 12 DE OCTUBRE FUNDACIÓN PROFESOR NOVOA SANTOS Georg-August-Universität Göttingen German Cancer Research Center (DKFZ) Göteborgs Universitet Gothenburg University Hadassah-Hebrew University Medical Center Hannover Medical School Harvard Medical School Harvard University Hebrew University of Jerusalem Heidelberg University Hospital/Department of Anesthesiology HHMI Hokkaido Institute for Pharmacy Benefit Co.,Ltd. ICGEB IDIBAPS (INSTITUT D´INVESTIGACIONS BIOMEDIQUES AUGUST PI I SUNYER) Ifremer Imperial College INCYTON GmbH Indiana University Pathology & Laboratory Medicine INRAE, AgroParisTech INSERM DRTLS INSERM U1232 CRCINA Institut D'investigacions Biomèdiques August Pi i Sunyer Institute Cochin Instituto de Investigación Hospital 12 de Octubre (i+12) Instituto Politécnico Nacional Internistische Praxis Dr. Hendricks IVIRMA Jagiellonian University Janssen R&D JAWAHARLAL NEHRU UNIVERSITY John Hunter Hospital Johns Hopkins School of Medicine Jozef Stefan Institute Karolinska Institutet Kent State University Kirin Holdigns Company Ltd. Konyang University Korea Brain Research Institute Kyungpook National University Hospital l'aquila/Department of Life, Health and Environmental Sciences Leibniz Institute on Aging Lions Eye Institute Lithuanian University of Health Sciences London School of Hygiene and Tropical Medicine Lunds Universitet Maastricht university Maine Medical Center Research Institute Massachusetts General Hospital Mayo Clinic Medical College of Wisconsin Medical Faculty of Heidelberg University Medical University Innsbruck Medizinische Universität Innsbruck Merrimack College Midwestern University MitoVasc Institute MitoVasc Institute, Angers University Hospital Center MITOVASC Mitolab / University of Angers MMCRI Moffitt Cancer Center Montpellier Cancer Research Institute Nagoya Univ Nagoya University Nanna Therapeutics Nastaran, University of Iowa National Heart, Lung, and Blood Institute National Institutes of Health National Neuroscience Institute Netherlands Institute for Neuroscience Neurology Research |
NIH/NINDS/SCN |
More Articles...
- Lack of mitochondria causes severe disease in children
- Mitochondrial RNA degradation is essential for life
- Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis
- The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan