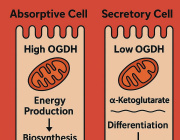
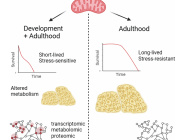
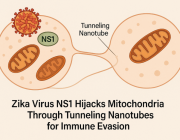
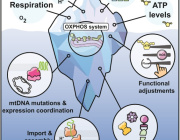
Lack of mitochondria causes severe disease in children
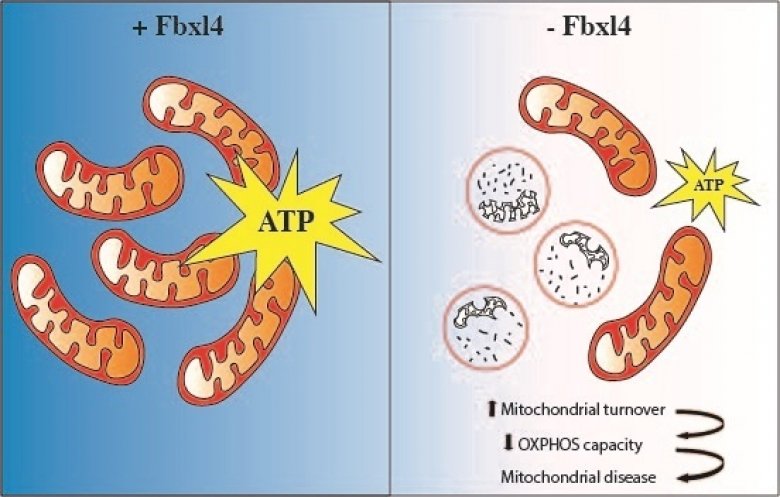
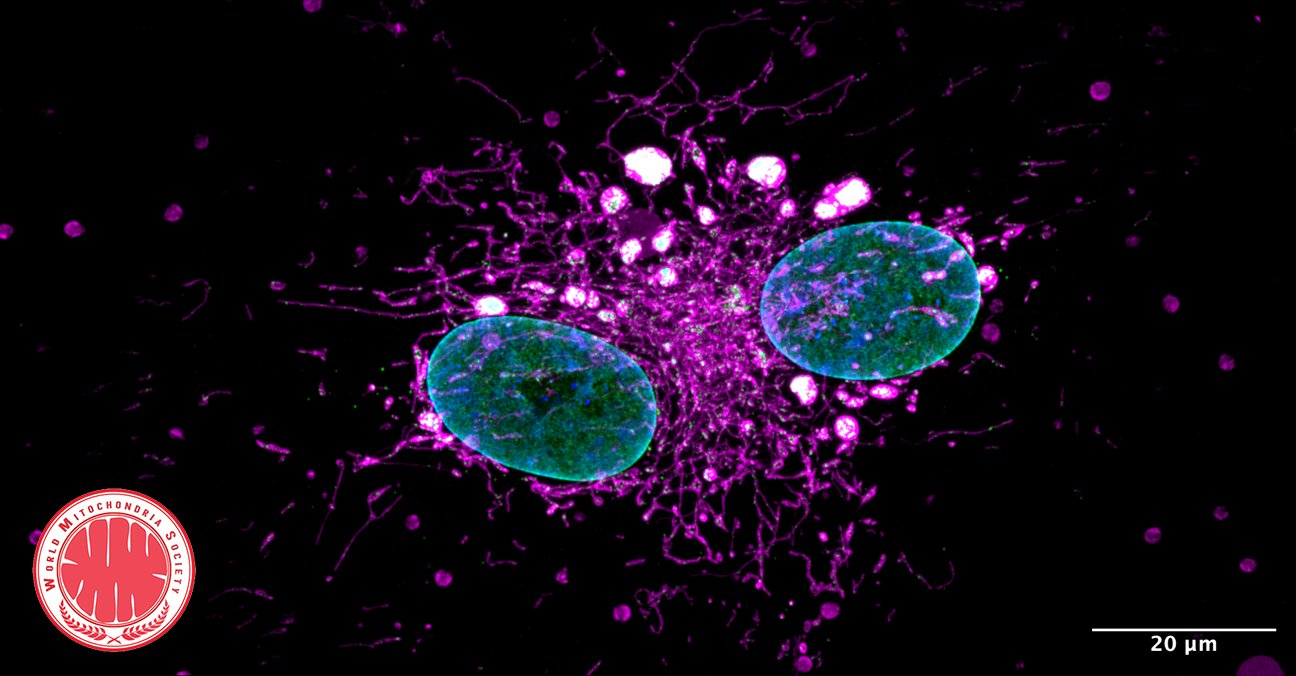
The absence of FBXL4 leads to degradation of mitochondria that results in decreased ATP (energy) production and mitochondrial disease. Credit : news.ki.se
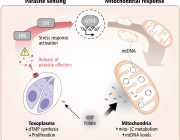
Researchers at Karolinska Institutet have discovered that excessive degradation of the power plants of our cells plays an important role in the onset of mitochondrial disease in children. These inherited metabolic disorders can have severe consequences such as brain dysfunction and neurological impairment. The study is published in EMBO Molecular Medicine.
“This is a completely new disease mechanism for mitochondrial disease which may provide a novel entry point for treating affected patients,” says Nils-Göran Larsson, professor at the Department of Medical Biochemistry and Biophysics, Karolinska Institutet, who led the study.
Mitochondrial diseases are inherited metabolic disorders that affect about 1 in 4,300 individuals and are caused by dysfunctional mitochondria. Mitochondria are the power plants of our cells and are crucial for converting energy derived from our food into the energy currency that drives the cell’s biochemical functions. Not surprisingly, organs that are mainly affected in patients are those with a high energy demand, such as the brain, heart, skeletal muscles, eyes and ears. In children, severe multisystem involvement and neurodegeneration are frequent manifestations.
Severe consequences
FBXL4 is a gene that is implicated in controlling mitochondrial function, and mutations in this gene are one of the most common causes of mitochondrial diseases. FBXL4 mutations have been linked to encephalopathy, a form of brain dysfunction causing neurological impairment. The manifestations are impaired cognitive function, developmental regression, epileptic seizures and other types of neurological deficits. Despite the severe consequences of FBXL4 mutations in humans, the function of the protein that FBXL4 codes for has remained poorly understood.
In the current study, researchers generated mice that lack FBXL4 and showed that these mice recapitulate important characteristics present in patients with FBXL4 mutations. They were able to demonstrate that the reduced mitochondrial function is caused by increased degradation of mitochondria via a process called autophagy.
Too few mitochondria in the tissues
In the absence of FBXL4, mitochondria are more frequently delivered to the lysosome, the recycling station of the cell that contains enzymes that break down organic compounds. FBXL4 thus acts as a break on mitochondrial degradation. Patients who lack FBXL4 have too few mitochondria in their tissues which leads to disease.
“Further studies are needed to explore the therapeutic potential of these findings, in particular whether inhibition of the degradation of mitochondria may provide a new treatment strategy,” says Nils-Göran Larsson.
The study was financed by several bodies, including the Swedish Research Council, the Knut and Alice Wallenberg Foundation, the European Research Council, the Swedish Cancer Society, and the ALF agreement between the Swedish government and the regional councils.
News source : https://news.ki.se/lack-of-mitochondria-causes-severe-disease-in-children
Authors : David Alsina, Oleksandr Lytovchenko, Aleksandra Schab, Ilian Atanassov, Florian A Schober, Min Jiang, Camilla Koolmeister, Anna Wedell, Robert W Taylor, Anna Wredenberg, Nils‐Göran Larsson
EMBO Mol Med (2020) 12: e11659https://doi.org/10.15252/emmm.201911659
Mitochondrial RNA degradation is essential for life
Credit : pubmed
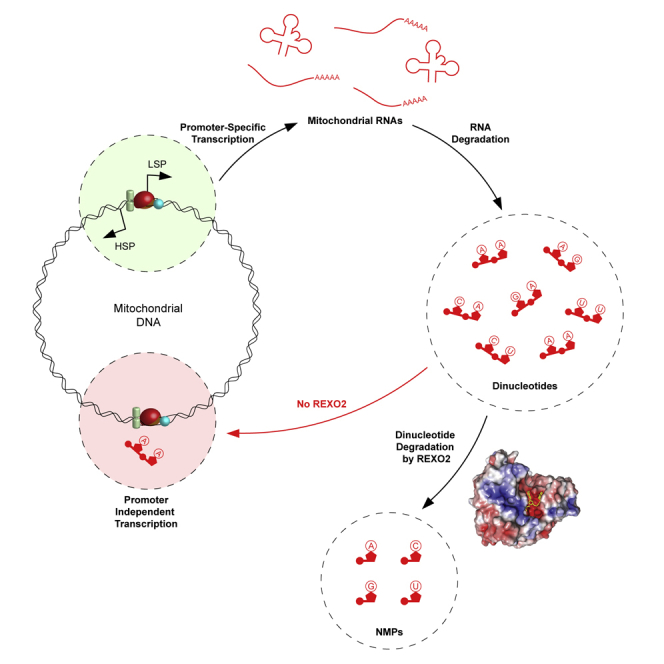
Researchers at Karolinska Institutet have discovered the essential role of the ribonuclease REXO2 in mitochondrial RNA degradation. The enzyme is essential for life, as a deficiency of it in mice has shown to be embryonic lethal. The study is published in the journal Molecular Cell.
We have asked two of the lead authors, Henrik Spåhr and Shan Jiang from the Nils-Göran Larsson group at the Department of Medical Biochemistry and Biophysics, about the most important results from their study.
“The mitochondria are the power plants of our cells and are essential for converting the energy in the food we eat to a useful cellular energy currency. Importantly, mitochondria contain their own genetic material, mitochondrial DNA (mtDNA). MtDNA is totally distinct from the majority of our genetic material that is located in the nucleus. Expression of mtDNA is essential for the energy conversion of the cell, and the first step is to copy (transcribe) mtDNA to mitochondrial RNA, which, in turn, is the template for making 13 proteins that all are critically important for the cellular energy conversion. Quite a bit is known about how mitochondrial RNA is formed, but critical steps in the degradation have remained unknown. In the present study, we have identified an enzyme that is required for the last step of RNA degradation in human mitochondria, called REXO2”, Henrik Spåhr explains.
“This enzyme selectively degrades nanoRNAs (dinucleotides) and it is essential for life, as REXO2 deficiency results in embryonic lethality in mice.”
News source : www.news.ki.se
Authors : Nicholls TJ, Spåhr H, Jiang S, et al. Dinucleotide Degradation by REXO2 Maintains Promoter Specificity in Mammalian Mitochondria. Mol Cell. 2019;76(5):784-796.e6. doi:10.1016/j.molcel.2019.09.010
The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan
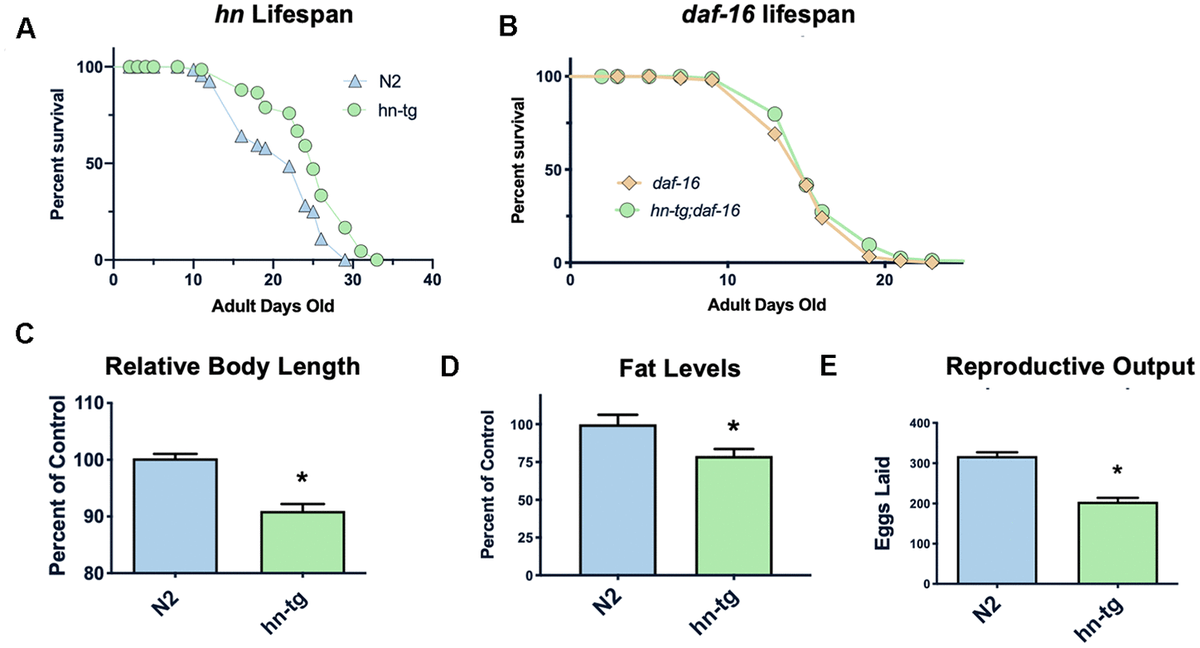 Figure 1. Humanin overexpression is sufficient to increase lifespan in C. elegans. Credit: aging-us.com
Figure 1. Humanin overexpression is sufficient to increase lifespan in C. elegans. Credit: aging-us.com
Humanin is a member of a new family of peptides that are encoded by short open reading frames within the mitochondrial genome. It is conserved in animals and is both neuroprotective and cytoprotective. Here we report that in C. elegans the overexpression of humanin is sufficient to increase lifespan, dependent on daf-16/Foxo. Humanin transgenic mice have many phenotypes that overlap with the worm phenotypes and, similar to exogenous humanin treatment, have increased protection against toxic insults. Treating middle-aged mice twice weekly with the potent humanin analogue HNG, humanin improves metabolic healthspan parameters and reduces inflammatory markers. In multiple species, humanin levels generally decline with age, but here we show that levels are surprisingly stable in the naked mole-rat, a model of negligible senescence. Furthermore, in children of centenarians, who are more likely to become centenarians themselves, circulating humanin levels are much greater than age-matched control subjects. Further linking humanin to healthspan, we observe that humanin levels are decreased in human diseases such as Alzheimer’s disease and MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke-like episodes). Together, these studies are the first to demonstrate that humanin is linked to improved healthspan and increased lifespan.
Article:
Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis
 Credit: multiplesclerosisnewstoday.com
Credit: multiplesclerosisnewstoday.com
Axonal loss is the key pathological substrate of neurological disability in demyelinating disorders, including multiple sclerosis (MS). However, the consequences of demyelination on neuronal and axonal biology are poorly understood. The abundance of mitochondria in demyelinated axons in MS raises the possibility that increased mitochondrial content serves as a compensatory response to demyelination. Here, we show that upon demyelination mitochondria move from the neuronal cell body to the demyelinated axon, increasing axonal mitochondrial content, which we term the axonal response of mitochondria to demyelination (ARMD). However, following demyelination axons degenerate before the homeostatic ARMD reaches its peak. Enhancement of ARMD, by targeting mitochondrial biogenesis and mitochondrial transport from the cell body to axon, protects acutely demyelinated axons from degeneration. To determine the relevance of ARMD to disease state, we examined MS autopsy tissue and found a positive correlation between mitochondrial content in demyelinated dorsal column axons and cytochrome c oxidase (complex IV) deficiency in dorsal root ganglia (DRG) neuronal cell bodies. We experimentally demyelinated DRG neuron-specific complex IV deficient mice, as established disease models do not recapitulate complex IV deficiency in neurons, and found that these mice are able to demonstrate ARMD, despite the mitochondrial perturbation. Enhancement of mitochondrial dynamics in complex IV deficient neurons protects the axon upon demyelination. Consequently, increased mobilisation of mitochondria from the neuronal cell body to the axon is a novel neuroprotective strategy for the vulnerable, acutely demyelinated axon. We propose that promoting ARMD is likely to be a crucial preceding step for implementing potential regenerative strategies for demyelinating disorders.
Article Reference: Licht-Mayer, S., Campbell, G.R., Canizares, M. et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol (2020). https://doi.org/10.1007/s00401-020-02179-x
One-time treatment generates new neurons, eliminates Parkinson's disease in mice
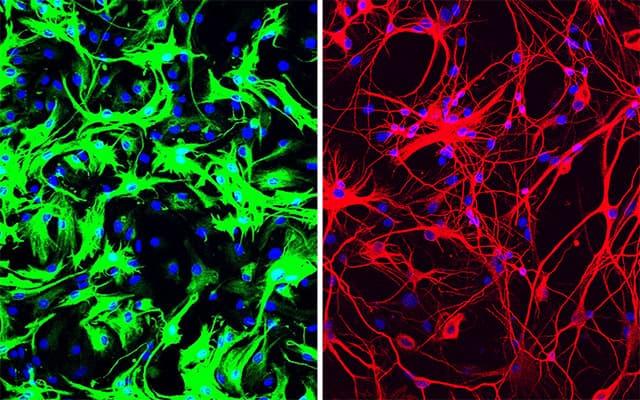 Left: mouse astrocytes (green) before reprogramming; Right: neurons (red) induced from mouse astrocytes after reprogramming with PTB antisense oligonucleotide treatment.
Left: mouse astrocytes (green) before reprogramming; Right: neurons (red) induced from mouse astrocytes after reprogramming with PTB antisense oligonucleotide treatment.
Credit: UC San Diego Health Sciences
Inhibiting a single gene converts many cell types directly into dopamine-producing neurons
Xiang-Dong Fu, PhD, has never been more excited about something in his entire career. He has long studied the basic biology of RNA, a genetic cousin of DNA, and the proteins that bind it. But a single discovery has launched Fu into a completely new field: neuroscience.
For decades, Fu and his team at University of California San Diego School of Medicine studied a protein called PTB, which is well known for binding RNA and influencing which genes are turned "on" or "off" in a cell. To study the role of a protein like PTB, scientists often manipulate cells to reduce the amount of that protein, and then watch to see what happens.
Several years ago, a postdoctoral researcher working in Fu's lab was taking that approach, using a technique called siRNA to silence the PTB gene in connective tissue cells known as fibroblasts. But it's a tedious process that needs to be performed over and over. He got tired of it and convinced Fu they should use a different technique to create a stable cell line that's permanently lacking PTB. At first, the postdoc complained about that too, because it made the cells grow so slowly.
But then he noticed something odd after a couple of weeks -- there were very few fibroblasts left. Almost the whole dish was instead filled with neurons.
In this serendipitous way, the team discovered that inhibiting or deleting just a single gene, the gene that encodes PTB, transforms several types of mouse cells directly into neurons.
More recently, Fu and Hao Qian, PhD, another postdoctoral researcher in his lab, took the finding a big step forward, applying it in what could one day be a new therapeutic approach for Parkinson's disease and other neurodegenerative diseases. Just a single treatment to inhibit PTB in mice converted native astrocytes, star-shaped support cells of the brain, into neurons that produce the neurotransmitter dopamine. As a result, the mice's Parkinson's disease symptoms disappeared.
The study is published June 24, 2020 in Nature.
"Researchers around the world have tried many ways to generate neurons in the lab, using stem cells and other means, so we can study them better, as well as to use them to replace lost neurons in neurodegenerative diseases," said Fu, who is a Distinguished Professor in the Department of Cellular and Molecular Medicine at UC San Diego School of Medicine. "The fact that we could produce so many neurons in such a relatively easy way came as a big surprise."
There are several different ways to mimic Parkinson's disease in mice. In this case, the researchers applied a dopamine look-a-like molecule to poison neurons that produce dopamine. As a result, the mice lose dopamine-producing neurons and develop symptoms similar to Parkinson's disease, such as movement deficiencies.
The treatment works like this: The researchers developed a noninfectious virus that carries an antisense oligonucleotide sequence -- an artificial piece of DNA designed to specifically bind the RNA coding for PTB, thus degrading it, preventing it from being translated into a functional protein and stimulating neuron development.
Antisense oligonucleotides, also known as designer DNA drugs, are a proven approach for neurodegenerative and neuromuscular diseases -- study co-author, Don Cleveland, PhD, pioneered the technology, and it now forms the basis for a Food and Drug Administration (FDA)-approved therapy for spinal muscular atrophy and several other therapies currently in clinical trials. Cleveland is chair of the Department of Cellular and Molecular Medicine at UC San Diego School of Medicine and member of the Ludwig Institute for Cancer Research, San Diego.
The researchers administered the PTB antisense oligonucleotide treatment directly to the mouse's midbrain, which is responsible for regulating motor control and reward behaviors, and the part of the brain that typically loses dopamine-producing neurons in Parkinson's disease. A control group of mice received mock treatment with an empty virus or an irrelevant antisense sequence.
In the treated mice, a small subset of astrocytes converted to neurons, increasing the number of neurons by approximately 30 percent. Dopamine levels were restored to a level comparable to that in normal mice. What's more, the neurons grew and sent their processes into other parts of brain. There was no change in the control mice.
By two different measures of limb movement and response, the treated mice returned to normal within three months after a single treatment, and remained completely free from symptoms of Parkinson's disease for the rest of their lives. In contrast, the control mice showed no improvement.
"I was stunned at what I saw," said study co-author William Mobley, MD, PhD, Distinguished Professor of Neurosciences at UC San Diego School of Medicine. "This whole new strategy for treating neurodegeneration gives hope that it may be possible to help even those with advanced disease."
What is it about PTB that makes this work? "This protein is present in a lot of cells," Fu said. "But as neurons begin to develop from their precursors, it naturally disappears. What we've found is that forcing PTB to go away is the only signal a cell needs to turn on the genes needed to produce a neuron."
Of course, mice aren't people, he cautioned. The model the team used doesn't perfectly recapitulate all essential features of Parkinson's disease. But the study provides a proof of concept, Fu said.
Next, the team plans to optimize their methods and test the approach in mouse models that mimic Parkinson's disease through genetic changes. They have also patented the PTB antisense oligonucleotide treatment in order to move forward toward testing in humans.
"It's my dream to see this through to clinical trials, to test this approach as a treatment for Parkinson's disease, but also many other diseases where neurons are lost, such as Alzheimer's and Huntington's diseases and stroke," Fu said. "And dreaming even bigger -- what if we could target PTB to correct defects in other parts of the brain, to treat things like inherited brain defects?
"I intend to spend the rest of my career answering these questions."
News Source: www.eurekalert.org
Article reference:
Qian, H., Kang, X., Hu, J. et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556 (2020). https://doi.org/10.1038/s41586-020-2388-4