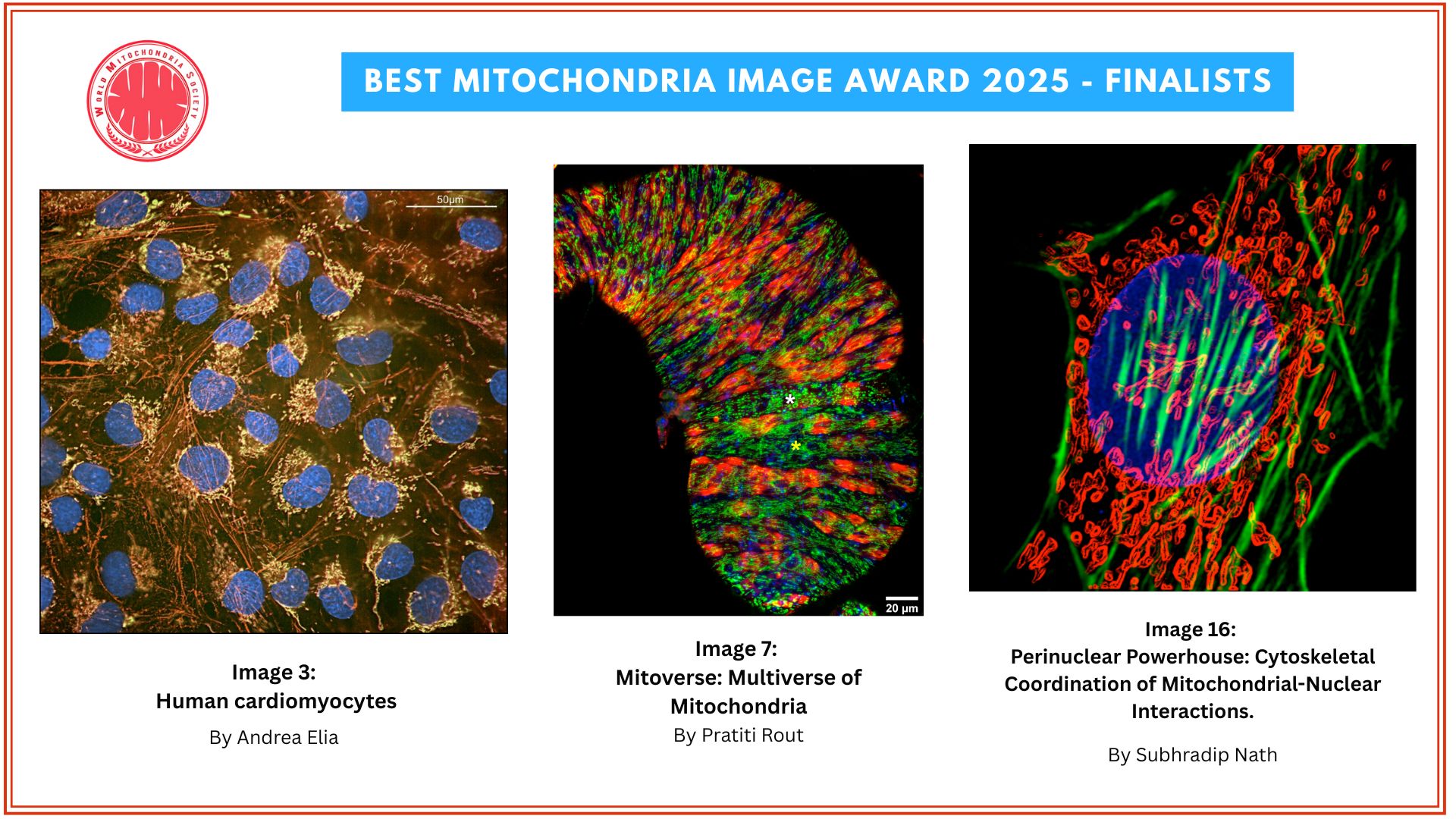
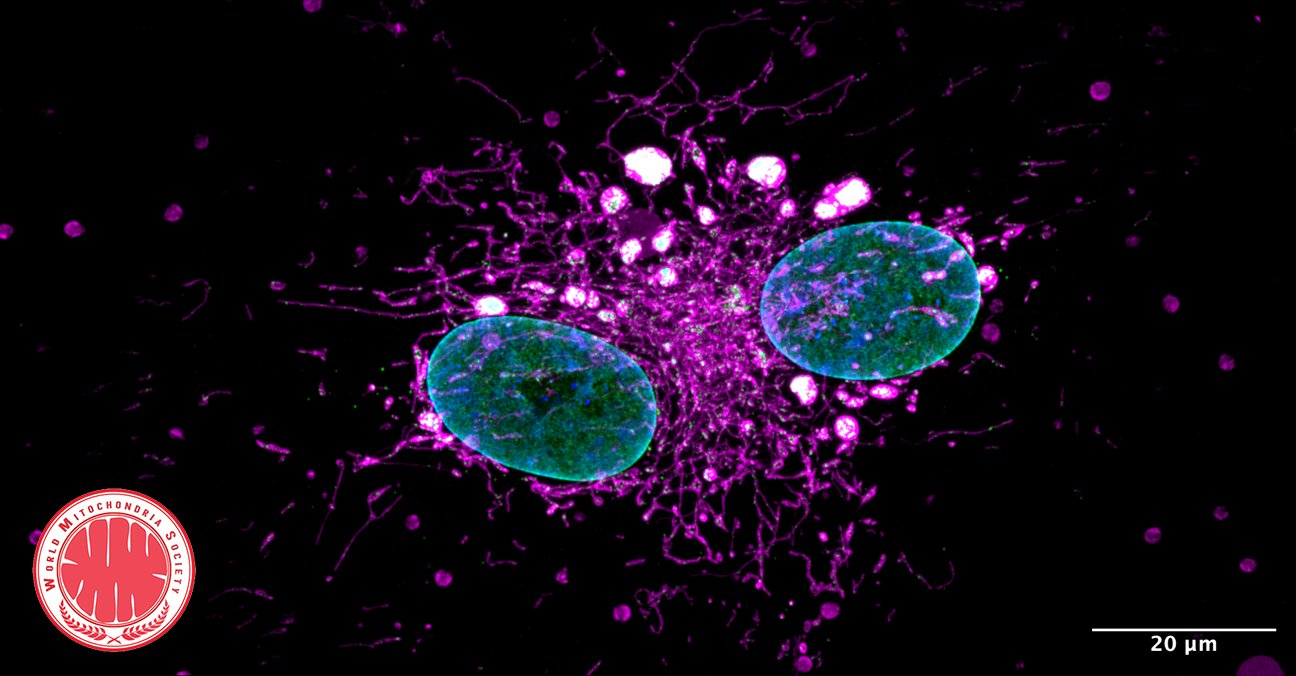
UCPH Researchers Prove Powerhouse Malfunction as the Major Cause of Parkinson’s Disease
UCPH Researchers Prove Powerhouse Malfunction as the Major Cause of Parkinson’s Disease
RESEARCH: The major cause of Parkinson’s Disease is a dysregulation of immune genes central for fighting against viruses, a new study reveals. Researchers from the University of Copenhagen show that this dysregulation leads to a malfunction in the cell’s powerhouse, which cannot produce sufficient energy for neurons to stay alive, causing them to gradually die.

Photo: Colourbox.
12,000 people in Denmark and 7 to 10 million people worldwide suffer from Parkinson’s Disease (PD). It is the second most common neurogenerative disorder of aging and the most common movement disorder, but the cause of the disease is largely unknown.
In a new study, researchers from the University of Copenhagen show that the most common form of the disease, encompassing 90 to 95 percent of all Parkinson’s Disease cases known as sporadic PD, is caused by a blockage of a pathway that regulates the nerve cell’s powerhouse, the mitochondria.
‘Just like when people eat, cells take what they need and get rid of the rest waste products. But if our brain cells have this specific kind of signaling blockage, it means that the powerhouse of the cell – mitochondria – cannot get cleaned up after being damaged’, explains corresponding author and group leader Professor Shohreh Issazadeh-Navikas at the Biotech Research & Innovation Centre.
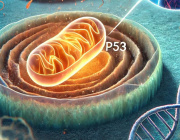
The blockage leads to an accumulation of high amounts of damaged mitochondria, while not being able to produce enough energy for the cells. It causes neurons to gradually die, which is the reason for the development of Parkinson’s Disease symptoms, and why it leads to dementia.
The blockage is caused by a dysregulation of the immune genes, more specifically a pathway called type 1 interferon, which is normally important for fight against viruses, but now we show that it is also responsible for regulating the energy supply of the nerve cells.
‘Every part of our body needs to be regulated. We get a signal to stop eating, when we are full, and the same thing happens everywhere else in our body. If we get an infection, parts of our body need to fight it and stop it from replicating. But when the infection is cleaned up, the signal should subside. This is the job of a protein called PIAS2. That causes the blockage of the type 1 interferon-pathway, and when the infection is over, the blockage should stop and go back to normal. But that does not seem to be the case in patients with Parkinson’s Disease. We further demonstrate that this dysregulation leads to a defect in the mitochondrial energy supply, as mentioned before’, says Shohreh Issazadeh-Navikas.
These pathways are very important for brain functions, but they are also associated with microbial and virus recognition. For example, they are very important for fighting COVID-19, and a mutation in the related gene has been shown to be linked to a deadly outcome after contracting COVID-19.
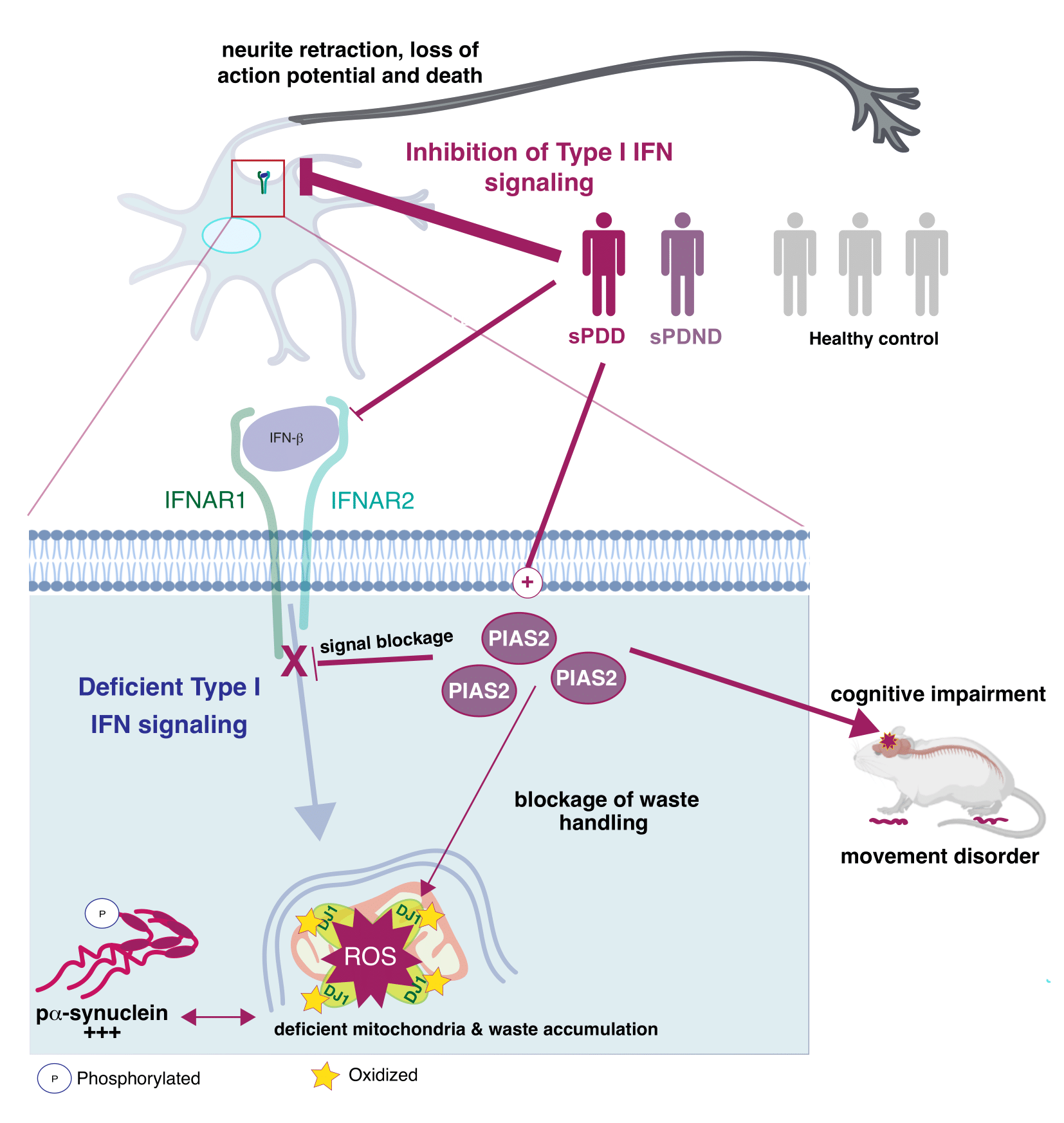
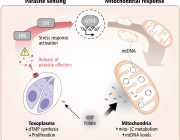
A detail look into what happens in the neurons. (See Illustration Above)
Combining Datasets for a Bigger Picture
The researchers combined and analyzed four data sets, which studied neurons from brains with Parkinson’s Disease and looked at what type of genes they express.
They then looked at which gene patterns were disturbed in patients with Parkinson’s Disease and especially those who had also developed PD with dementia.
In order to test the results, the major findings of the combined data was tried in three different mouse models using a negative regulator of the type I interferon pathway, PIAS2, which had been identified from the patients study as one of the key proteins linked to the progression of Parkinson’s Disease and dementia.
‘We show that a high accumulation of the PIAS2-protein is what is causing the blockage in the pathway, which should have activated the processes responsible for removing damaged protein and mitochondrial garbage’, says Shohreh Issazadeh-Navikas.
‘The accumulation of damaged mitochondrial mass further leads to increase of other toxic proteins. So when we compare patients to same-aged healthy patients without Parkinson’s Disease, we see that this PIAS2-protein is highly expressed in the neurons, which is why this pathway should be evaluated for potential roles in the other forms of familial Parkinson’s Disease that we have not studied here.’
The researchers hope the study will encourage research to counteract the pathway blockage, which could have a beneficial impact on the disease and towards preventing dementia.
In the next stages, the Shohreh Group will study how the pathway contributes to neuronal homeostasis and survival, as well as how its dysregulation causes neuronal cell death.
News Source: University of Copenhagen
It’s True: Stress Does Turn Hair Gray (And It’s Reversible)

Legend has it that Marie Antoinette’s hair turned gray overnight just before her beheading in 1791.
Though the legend is inaccurate—hair that has already grown out of the follicle does not change color—a new study(link is external and opens in a new window) from researchers at Columbia University Vagelos College of Physicians and Surgeons is the first to offer quantitative evidence linking psychological stress to graying hair in people.
And while it may seem intuitive that stress can accelerate graying, the researchers were surprised to discover that hair color can be restored when stress is eliminated, a finding that contrasts with a recent study in mice that suggested that stressed-induced gray hairs are permanent.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” Picard says.
“Our data add to a growing body of evidence demonstrating that human aging is not a linear, fixed biological process but may, at least in part, be halted or even temporarily reversed.”
Studying hair as an avenue to investigate aging
“Just as the rings in a tree trunk hold information about past decades in the life of a tree, our hair contains information about our biological history,” Picard says. “When hairs are still under the skin as follicles, they are subject to the influence of stress hormones and other things happening in our mind and body. Once hairs grow out of the scalp, they harden and permanently crystallize these exposures into a stable form.”
Though people have long believed that psychological stress can accelerate gray hair, scientists have debated the connection due to the lack of sensitive methods that can precisely correlate times of stress with hair pigmentation at a single-follicle level.
Splitting hairs to document hair pigmentation
Ayelet Rosenberg, first author on the study and a student in Picard’s laboratory, developed a new method for capturing highly detailed images of tiny slices of human hairs to quantify the extent of pigment loss (graying) in each of those slices. Each slice, about 1/20th of a millimeter wide, represents about an hour of hair growth.
“If you use your eyes to look at a hair, it will seem like it’s the same color throughout unless there is a major transition,” Picard says. “Under a high-resolution scanner, you see small, subtle variations in color, and that’s what we’re measuring.”
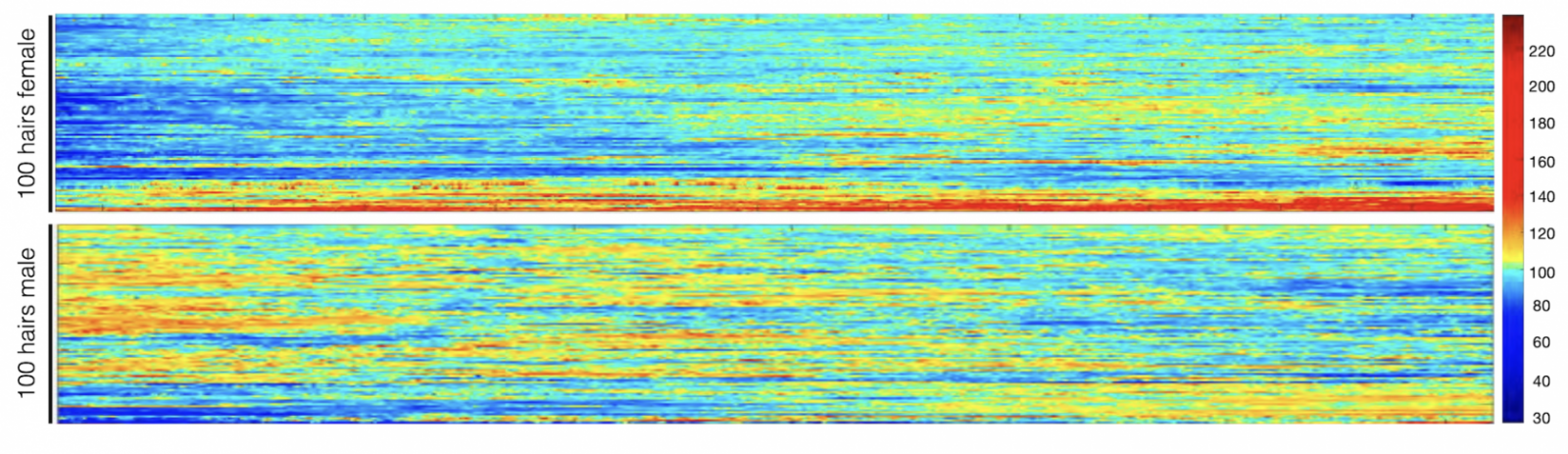 Hair pigmentation patterns of 100 hairs from a male and female study participant. Darker hair colors represented in red; lighter in blue. Image from Rosenberg et al. (2021).
Hair pigmentation patterns of 100 hairs from a male and female study participant. Darker hair colors represented in red; lighter in blue. Image from Rosenberg et al. (2021).
The researchers analyzed individual hairs from 14 volunteers. The results were compared with each volunteer’s stress diary, in which individuals were asked to review their calendars and rate each week’s level of stress.
The investigators immediately noticed that some gray hairs naturally regain their original color, which had never been quantitatively documented, Picard says.
When hairs were aligned with stress diaries by Shannon Rausser, second author on the paper and a student in Picard’s laboratory, striking associations between stress and hair graying were revealed and, in some cases, a reversal of graying with the lifting of stress.
“There was one individual who went on vacation, and five hairs on that person’s head reverted back to dark during the vacation, synchronized in time,” Picard says.
Blame the mind-mitochondria connection
To better understand how stress causes gray hair, the researchers also measured levels of thousands of proteins in the hairs and how protein levels changed over the length of each hair.
Changes in 300 proteins occurred when hair color changed, and the researchers developed a mathematical model that suggests stress-induced changes in mitochondria may explain how stress turns hair gray.
“We often hear that the mitochondria are the powerhouses of the cell, but that’s not the only role they play,” Picard says. “Mitochondria are actually like little antennas inside the cell that respond to a number of different signals, including psychological stress.”
The mitochondria connection between stress and hair color differs from that discovered in a recent study of mice, which found that stress-induced graying was caused by an irreversible loss of stem cells in the hair follicle.
“Our data show that graying is reversible in people, which implicates a different mechanism,” says co-author Ralf Paus, PhD, professor of dermatology at the University of Miami Miller School of Medicine. “Mice have very different hair follicle biology, and this may be an instance where findings in mice don’t translate well to people.”
Hair re-pigmentation only possible for some
Reducing stress in your life is a good goal, but it won’t necessarily turn your hair to a normal color.
“Based on our mathematical modeling, we think hair needs to reach a threshold before it turns gray,” Picard says. “In middle age, when the hair is near that threshold because of biological age and other factors, stress will push it over the threshold and it transitions to gray.
“But we don’t think that reducing stress in a 70-year-old who’s been gray for years will darken their hair or increasing stress in a 10-year-old will be enough to tip their hair over the gray threshold.”
News source: www.cuimc.columbia.edu/
New warning system discovered in the immune defence
Researchers at Linköping University in Sweden have discovered a previously unknown warning system that contributes to the body's immune system. Mitochondria in the white blood cells secrete a web of DNA fibres that raises the alarm.
Credit: Reproduced with permission from Proceedings of the National Academy of Sciences USA
Introduction is: White blood cells are major components of the body’s immune defence, and the research group has shown that several types of these cells react against small DNA fragments that are similar to the DNA from bacteria and viruses. The white blood cells spray out a web consisting of mitochondrial DNA (mtDNA) strands. Mitochondria are present in all cells and normally produce the energy needed by the cell, by burning sugar and fat to form water and carbon dioxide.
The web that the mitochondria release sends signals to the surrounding cells that the body is under attack, and cause other white blood cells to release a signal substance known as “interferon type 1”. This substance helps the immune system to combat the infection.
Conclusion is: High levels of interferon type 1, the signal substance activated by the mtDNA webs, occur in several autoimmune diseases and several types of cancer. The researchers believe that it may be possible to quantify the secreted mtDNA molecules and interpret the warning signals, and in this way understand these diseases better.
Full Article: https://www.eurekalert.org/pub_releases/2018-01/lu-nws011218.php
Targeting Mitochondria 2021 Congress
October 27-29, 2021
Berlin, Germany & Virtual Congress
www.targeting-mitochondria.com
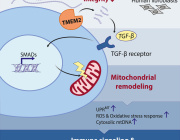
Age-Related Mitochondrial Dysfunction as a Key Factor in COVID-19 Disease
The Scientific Committee would like to share this excellent article written by Guillermo López-Lluch and al. on Age-Related Mitochondrial Dysfunction as a Key Factor in COVID-19 Disease. This article will be presented in Targeting Mitochondria Congress 2021.
The Scope is that Coronavirus Disease 2019 (COVID-19) is a complex respiratory and thrombogenic disease caused by a new strain of coronavirus known as Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Many of the patients infected with SARS-CoV-2 are asymptomatic or show low intensity symptoms. However, around 20% of the patients, mainly elderly people, manifest severe symptoms and high mortality ratio. COVID-19 predominant symptoms are high fever, intense cough, pneumonia and myalgia, mainly leading to acute respiratory distress syndrome (ARDS). Diarrhea and nausea can precede fever or respiratory symptoms. These symptoms can be aggravated in senior patients aged more than 70 years.
To conclude, we postulate here that the maintenance of mitochondrial health through healthy life habits, pharmacological compounds o dietary supplements able to improve mitochondrial activity, dynamics and turnover would reduce the levels of inflammatory cytokines and the severity of COVID-19 and other respiratory diseases such as seasonal flu. These and other “geroprotective” treatments must be used to treat or prevent COVID-19. Interventions and compounds able to improve mitochondrial health in the elderly would reduce or even eliminate the cytokine storm induced by SARS-CoV-2 infection and, therefore, reduce the grade of ARDS associated with COVID-19.
Article link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7648491/
Targeting Mitochondria 2021 Congress
October 27-29, 2021
Berlin, Germany & Virtual Congress
www.targeting-mitochondria.com
Mitochondrial Functioning and the Relations among Health, Cognition, and Aging: Where Cell Biology Meets Cognitive Science
The Scientific Committee of the World Mitochondria Society would like to share this article by David C. Geary, Department of Psychological Sciences, University of Missouri on Mitochondria, general intelligence, health and aging.

Credit: https://www.mydocuc.com/health-aging/
The Scope is: Performance in one cognitive domain, such as attentional control, is positively correlated with performance in all other cognitive domains, such as reading comprehension, and performance in all of these domains is correlated with current and predictive of later health outcomes. These relations suggest a common biological mechanism that contributes to cognition and health; moreover, this mechanism has been linked to systematic and parallel declines in cognition and health with normal aging. Mitochondrial functioning, including contributions to cellular energy production, control of oxidative stress, immunity, and intracellular signaling (among others), is well situated to explain at least some of these links.
The Conclusion is: There is now consistent evidence that various mitochondrial functions, including energy production, control of oxidative stress, and intracellular signalling, among others, contribute to the well-documented relations between cognition, health, and aging. These relations provide a natural link between research in cell biology and cognitive science. Advances in the latter are potentially useful for the development of measures that will be the most sensitive to age- or disease-related disruptions of mitochondrial functions or therapeutic enhancement of these functions and their potential influence on cognition.
DOI: https://doi.org/10.3390/ijms22073562
Targeting Mitochondria 2021 Congress
October 27-29, 2021
Berlin, Germany & Virtual Congress
www.targeting-mitochondria.com