The mitochondrial protein Opa1 promotes adipocyte browning that is dependent on urea cycle metabolites
Submitochondrial localization of Mgm1/OPA1 protein as determined by immuno-electron microscopy. Credits: Loss of the Intermembrane Space Protein Mgm1/OPA1 Induces Swelling and Localized Constrictions along the Lengths of Mitochondria - Scientific Figure on ResearchGate.
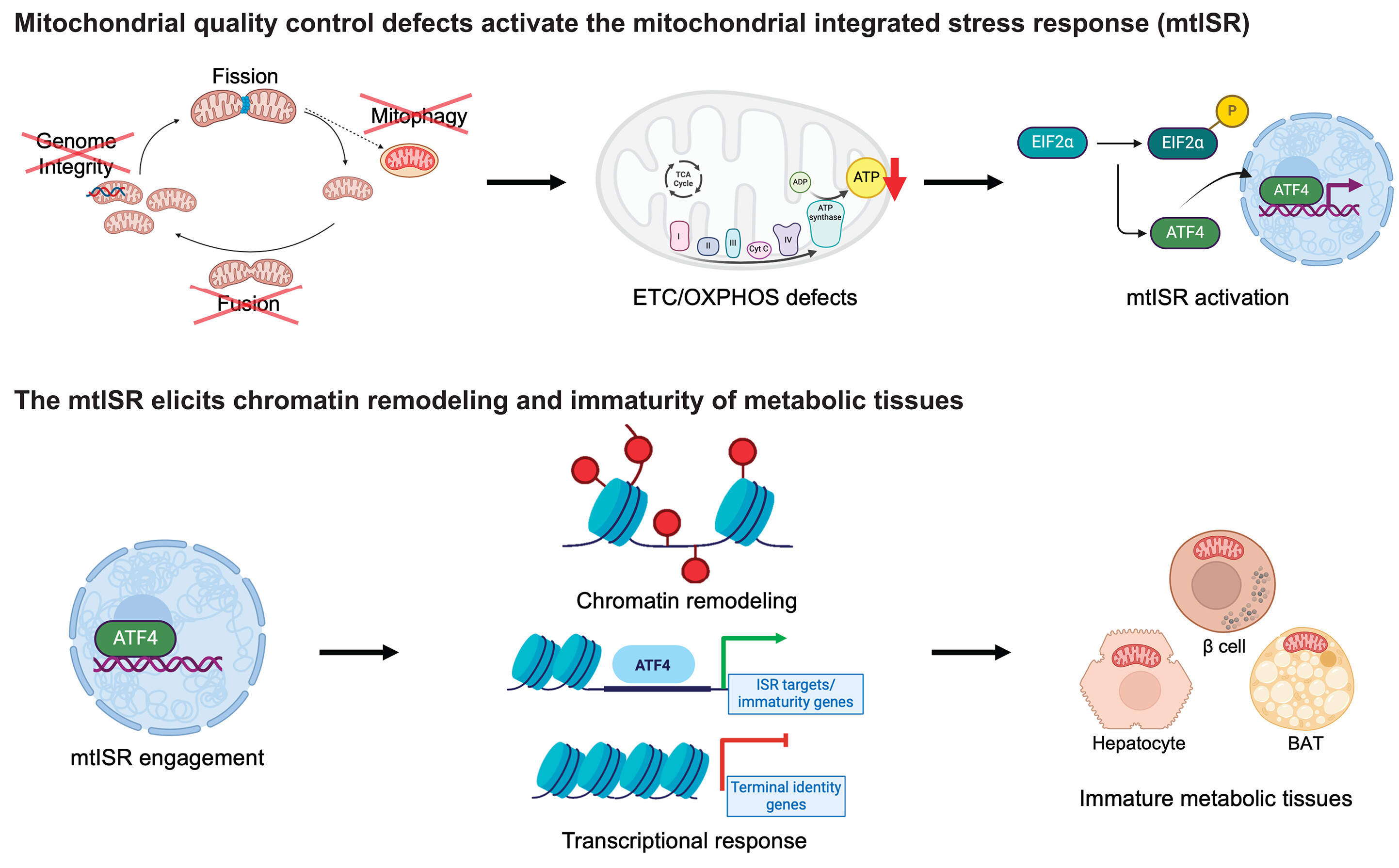
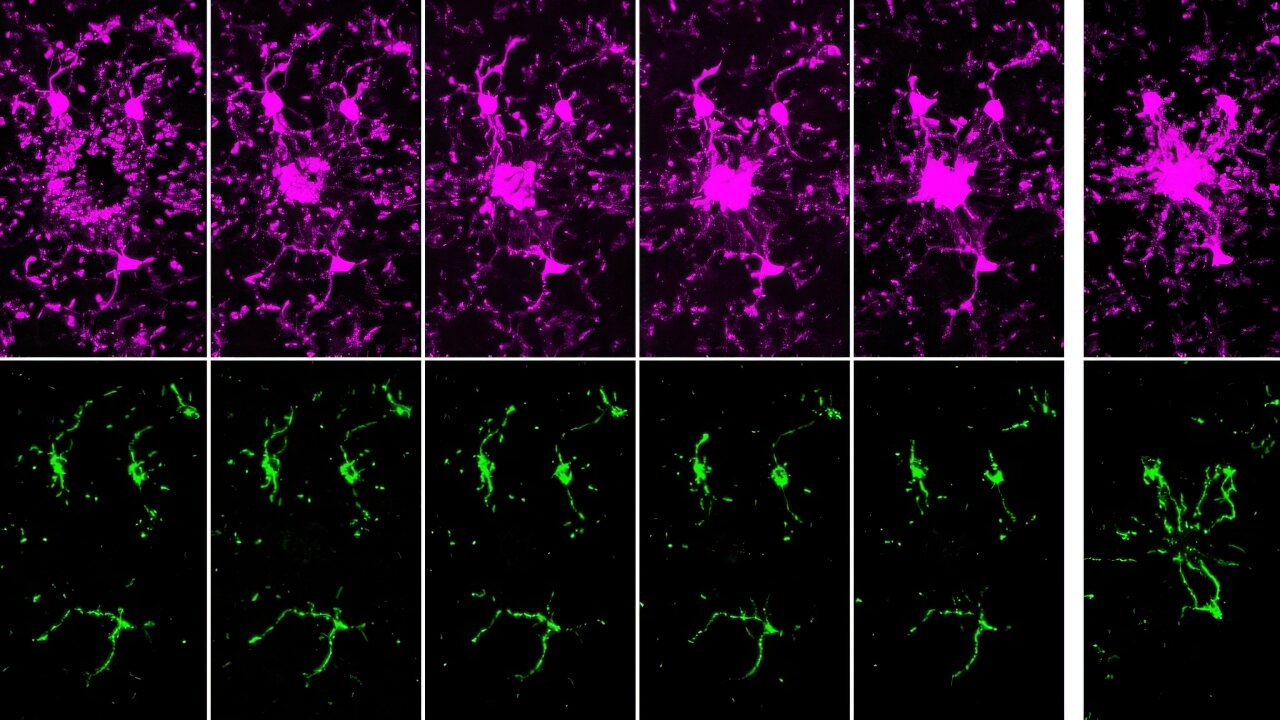
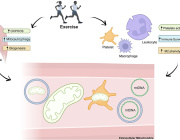
The conversion of white to brown/beige adipocytes is a possible therapeutic strategy for tackling the current obesity epidemics. Mitochondria are known to be key for energy dissipation in brown fat, but it is unknown if they can drive adipocyte browning. In this study, they showed that the mitochondrial cristae biogenesis protein optic atrophy 1 (Opa1) facilitates cell-autonomous adipocyte browning.
They reported that, Adipose tissue OPA1 levels were reduced in two cohorts of patients with obesity. Also, the overexpression of Opa1 in mouse favored white adipose tissue expandability as well as browning, improving glucose tolerance and insulin sensitivity.
They identified, using transcriptomics and metabolomics analyses, Jumanji family chromatin remodeling protein Kdm3a and urea cycle metabolites, including fumarate, as effectors of Opa1-dependent browning.
They indicated, using flux analyses, that Opa1-dependent fumarate accumulation is dependent on the urea cycle.
In conclusion, they stated that the urea cycle links the mitochondrial dynamics protein Opa1 to white adipocyte browning.
Authors: Bean, C., Audano, M., Varanita, T. et al.