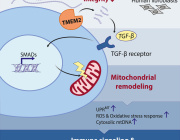
Can Vitamins and Supplements Help Patients with Mitochondrial Disease?
Defects in mitochondria, the tiny structures that power our cells by functioning as biological batteries, cause an array of complex, often life-threatening disorders that can affect any and all organs and systems. In the absence of validated, effective drug treatments, patients with mitochondrial disease often take a variety of vitamins and supplements, substances that are largely unstandardized, unregulated and unproven.
Experts in mitochondrial medicine propose to remedy that situation, calling for systematic scientific studies in cells and animals to lay the foundation for clinical trials of precise nutritional interventions for patients with energy deficiency diseases.
“We’re aiming to raise the bar for clinical treatments,” said Marni J. Falk, MD, Executive Director of the Mitochondrial Medicine Frontier Program at Children’s Hospital of Philadelphia (CHOP). Falk co-authored a new analysis of nutritional interventions for mitochondrial disorders published Nov. 3 in the Annual Review of Pathology: Mechanisms of Disease. “Our major objectives were to review the basic scientific evidence for compounds already being used in mitochondrial disease patients and to advocate a framework for rigorously evaluating their safety and efficacy in this population.”
The review article represents the collaborative effort of expert co-authors from eight centers, including first author Adam J. Kuszak, PhD, of the Office of Dietary Supplements of the National Institute of Health (NIH). The current effort grew out of a 2014 NIH meeting focused on developing an evidence base for nutritional interventions in primary mitochondrial disorders.
“Our analysis made it clear how much more we need to learn about developing effective nutritional treatments for mitochondrial disease,” said co-author Zarazuela Zolkipli-Cunningham, MBChBD, a neuromuscular specialist and attending physician in CHOP’s Mitochondrial Medicine Frontier Program. “There’s a large gap between the compounds that patients are routinely using and the degree to which those compounds have been scientifically tested.”
For instance, Zolkipli-Cunningham pointed to an “astounding variety” of the supplement coenzyme Q10 (CoQ10), sold over the counter in diverse versions and dosages. It is marketed as an antioxidant to reduce biological damage from reactive oxidant molecules.
However, she pointed out, there is no definitive evidence for health benefits from CoQ10. Moreover, there are no standardized formulations for this supplement, so patients may receive widely varying ingredients from one product to another. A third consideration is that a given supplement may act differently in a healthy consumer than in an individual with a mitochondrial disorder, because defects in mitochondria have wide-ranging effects on cellular function. Finally, supplements may act very differently across different subtypes of mitochondrial disease.
“Anything that affects cellular function is biologically acting as a drug, whether you obtain it from a pharmacy or a health food store,” said Falk. “However, unlike prescription medications, which are closely regulated and standardized by the U.S. Food and Drug Administration, vitamins, dietary supplements, and medical foods are considered in our country to be in a separate regulatory category with much less stringent requirements. Their manufacturing standards are not as tightly regulated, and their claims are limited to optimizing general public health, not to treating specific diseases. So we know a lot less about their safety and efficacy in patients.”
To read the complete news, please follow the link to original news: http://www.chop.edu/news/can-vitamins-and-supplements-help-patients-mitochondrial-disease
Mitochondrial replacement moratorium should be reconsidered, researchers say
Mothers with mitochondrial DNA mutations often give birth to children who face incurable and fatal illnesses. But a much-studied form of mitochondrial replacement (MR) could prevent the transmission of such diseases from mothers to children, researchers say.
For that reason, two researchers argue that the U.S. moratorium that includes MR should be reconsidered through a process that engages the public, medical professionals, the U.S. Food and Drug Administration and Congress.
The authors -- Eli Adashi, a professor of medical science at Brown University's Warren Alpert Medical School, and Harvard Law School professor I. Glenn Cohen -- make their case in a March 2018 commentary in Obstetrics & Gynecology.
Such a process could clarify the benefits of the procedure -- namely, the births of healthy children -- and decouple it from misplaced concerns about genetic editing of embryos, the authors wrote. MR therapy simply replaces mutation-bearing mitochondria in oocytes (unfertilized, un-implanted eggs) with donated mutation-free mitochondria.
"A thousand children are born every year in the U.S. with serious, life-threatening issues that in a better world could be prevented by mitochondrial replacement," Adashi said. "While I have every respect for the sanctity of life, this issue is not about the sanctity of life. There is an inherent hypocrisy in holding this procedure hostage at the expense of 1,000 children each year who are doomed to die a painful death. There is nothing anti-life about the procedure, because no embryo is destroyed, and the life of baby is saved."
The moratorium
In 2016, legislation was passed that prohibits U.S.-based research in which a human embryo is intentionally created or modified, the study notes. While MR does not modify or "enhance" the nuclear genome, according to Adashi, replacing mutation-bearing mitochondria with donated mutation-free mitochondria falls under the general category of procedures prohibited by the moratorium.
In their commentary, Adashi and Cohen point out that the authors of the legislation are anonymous and that no congressional hearings, floor discussions or public engagement took place before its passage.
The authors surmise that the legislation may have been intended primarily to prevent embryo loss, a concern that does not apply to MR. A public process of reevaluating MR's inclusion in the moratorium could help to clarify that MR takes place before an embryo exists. The donated mitochondria are placed within unfertilized eggs, which can then be fertilized so that women can give birth to genetically related, disease-free children.
It is possible, Adashi said, that a misunderstanding of the sweeping nature of the legislation inadvertently bars the procedure.
"Mitochondrial replacement is best viewed as life-enhancing in its outlook by dint of its capacity to alleviate human suffering in a context where no other option exists," the authors wrote.
Impact of the moratorium
The moratorium deprives affected American families of the opportunity to prevent inherited, incurable and agonizing mitochondrial disease in their children, the authors contend.
Mitochondrial diseases include Leigh syndrome, a progressive and fatal disorder characterized by lesions on the brain that may lead to heart, kidney, vision and breathing complications, and Alpers Disease, a neurologic illness that causes seizures, dementia, spasticity, blindness, liver dysfunction and cerebral degeneration.
The moratorium may also induce American families to seek care outside of the country, according to Adashi and Cohen. They noted that a U.S.-led team in Mexico may have prevented Leigh syndrome in a child by replacing the mutation-bearing mitochondria of oocytes with donated mutation-free oocytes.
"This development calls into question the regulatory utility of a national moratorium in a globalized world wherein cross-border care is increasingly prevalent," Adashi and Cohen wrote in the study. It also creates risks, the authors assert, because there is no FDA oversight of these procedures that take place outside the U.S. border.
A path forward
Adashi and Cohen recommend that a coalition of patient and advocacy groups, medical professionals and legislators convene congressional hearings on the prevention of mitochondrial diseases. They also suggest convening a public meeting of the FDA's Cellular, Tissue and Gene Therapies Advisory Committee, charged with regulating reproductive technologies, to review the state-of-the-art procedure.
They also recommend stringent FDA oversight, the conditional approval of biologic licensing applications, clinic-specific licensing, possible sunset contingency provisions, and long-term intergenerational follow-up of the children of mothers who undergo mitochondrial replacement to determine the continuing safety and efficacy of the intervention.
In the U.K., a careful 15-year vetting process resulted in a vote in Parliament that approved MR under stringent regulatory oversight. In the U.S., on the other hand, "Congress legislated a statute that prohibits the FDA from adjudicating research into a range of procedures hereby treating the issue with a broad brush," Adashi said.
"They spent 15 years studying it -- the science, the safety, the ethics -- and they asked the British public what they thought," he added. "Now MR is legal but regulated by an agency that has been proceeding very cautiously, with just one clinic licensed to perform the procedure."
What this means, Adashi said, is that parents who are at risk for transmitting mitochondrial disease to their children may now undergo MR and have children who are not born with agonizing and untreatable diseases. American parents, Adashi and Cohen wrote in the commentary, deserve nothing less.
News source: https://www.sciencedaily.com
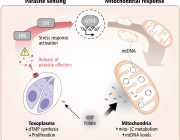
Cells stressed out? Make mitochondria longer
Scientists at The Scripps Research Institute (TSRI) have discovered a new pathway in cells that promotes mitochondrial function during times of stress, a response that can guard against disease as we age.
In response to stress, rather than churn out misshapen proteins, our cells activate protective pathways that take an even more dramatic response -- shutting down protein production entirely. Researchers show that along with this shutdown comes an odd change in shape of organelles called mitochondria, which are responsible for generating cellular energy. Instead of looking like tiny lima beans, mitochondria start to stretch out like noodles.
"Just a couple hours of not making proteins seems to be enough to remodel the mitochondria, and they can stay that way for hours," says Luke Wiseman, PhD, associate professor at TSRI and senior author of the new study, published this week in the journal Cell Reports. "That seems to be a protective way to promote mitochondrial function during the early stages of stress."
The new study offers a closer look at a stress-response pathway in cells called the Unfolded Protein Response (UPR). The UPR has several "branches" that regulate different cellular functions. The Wiseman lab focuses on how stress in a compartment of cells called the endoplasmic reticulum (ER) affects mitochondrial shape and function.
An important player in this response is a sensor/initiator of the UPR called PERK. Wiseman describes the PERK branch as a finely tuned signaling pathway. Without enough PERK signaling, the mitochondria can go haywire in times of stress and significantly challenge cellular function. But if this pathway is hyperactivated, the cell self-destructs.
As we age however, it becomes difficult for the system to maintain this balance. "When you're older, little problems can become bigger problems because the PERK pathway isn't as good at responding," Wiseman says.
Previous research shows that in times of stress, PERK has an important role in regulating many aspects of mitochondrial function including preventing the mitochondrial accumulation of misshapen proteins in response to ER stress. This new study shows that shutting down protein production through activation of PERK also influences mitochondrial shape by increasing its length. Changes in mitochondrial shape are known to influence mitochondrial function, indicating that this is a mechanism to adapt mitochondrial function during ER stress.
The next question for the team was whether this shutdown and remodeling was helping or hurting cells. The mitochondria's main role is to produce energy for the cell, so the researchers measured energy output to see how well mitochondria were functioning after cells experienced ER stress.
They found that shutting down protein production and remodeling the mitochondria did make a difference. "We were able to able to show a protective effect, where mitochondrial energy production was protected due to increased mitochondrial length" says Justine Lebeau, PhD, research associate at TSRI and co-first author of the study.
The researchers suspect that this whole system evolved to give cells a way to respond to stress very quickly, when they just don't have time to make a batch of protective proteins.
"Blocking protein synthesis -- and promoting cellular energy levels by regulating mitochondrial shape -- seems to be an effective way of combatting stress over shorter time scales," says Aparajita Madhavan, graduate student at TSRI and co-first author of the study.
Wiseman thinks defects in PERK sensitivity/activation caused by aging or mutations might hinder this protective regulation of mitochondria. He says defects in PERK signaling are implicated in many diseases that also include mitochondrial dysfunction, such as diabetes, heart disease, and neurodegenerative disorders such as Alzheimer's and Parkinson's disease. He hopes the new work could point to a way to target this aspect of PERK signaling to correct mitochondria defects that cause disease.
News source: https://www.sciencedaily.com/releases/2018/03/180314120214.htm
'Natural insecticide' kills advanced prostate cancer cells: A Mitochondria issue

One of the hallmarks of advanced prostate cancer is a faulty PTEN tumor suppressor gene. Now, after screening compounds for their effect on cells lacking PTEN, scientists have discovered that a natural insecticide called deguelin can kill such cells by disrupting their energy supply.
Deguelin belongs to a class of drugs known as mitochondrial inhibitors. The drugs block the action of mitochondria.
Mitochondria are the tiny compartments inside cells that convert glucose in the cell into molecules of adenosine triphosphate (ATP), which serve as units of energy for fueling the various workings of the cell.
Scientists at Cold Spring Harbor Laboratory in New York found that treating cells lacking PTEN with some types of mitochondrial inhibitor caused the cells to use glucose from their environment to make ATP and then transport it into their mitochondria to preserve them.
It is as though cells without PTEN, explains study leader Lloyd Trotman, a professor at Cold Spring Harbor Laboratory, are driven to "consume vast quantities of glucose" to help their mitochondria survive. They do this to the point where they run out of fuel and die.
The researchers describe their work — which included the use of a genetic mouse model of metastatic prostate cancer that was developed by Prof. Trotman's group — in a paper now published in the journal Cell Reports.
They suggest that their findings show that, at the right dose, certain mitochondrial inhibitors such as deguelin — and another that they identified called rotenone — may be able to kill prostate cancer cells without harming healthy cells.
However, they also note that the timing and conditions have to be just right – for example, the drug would not work if glucose levels are high.
"The hope is," Prof. Trotman explains, "that carefully timed administration of these drugs can generate a much better window of selective killing."
News source: https://www.medicalnewstoday.com
Photo Credit: medicalnewstodat
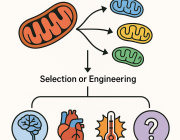
New Method Improves Delivery of Healthy Mitochondria To Cells Where It Is Dysfunctional, Study Shows
South Korean researchers have developed a simple way to deliver healthy mitochondria to cells where it is dysfunctional, rescuing the cells’ energy levels and metabolic function.
The study, “Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function,” was published in the journal Scientific Reports.
Defects in mitochondria, the small organelles that are the cell’s powerhouses, contribute to several health problems, including aging, cancer, metabolic disorders and neurodegenerative diseases.
Scientists have developed therapies aimed at rescuing mitochondria’s function. But they are limited to cases where a gene mutation underlies the defects.
Researchers have begun exploring a new strategy — replacing damaged mitochondria with healthy ones.
But a problem scientists have faced is that “mitochondrial transfer methods are inefficient and time-consuming,” the South Korean researchers wrote.
They came up with a new way to deliver healthy mitochondria. The first step is to mix healthy mitochondria with the cells where they need to go. The next step is spinning the mixture — a process called centrifugation — to combine the mitochondria and cells.
The team spun a mixture of mitochondria from human umbilical cord-derived mesenchymal stem cells and cells that needed healthy mitochondria.
They tried the process with more than one type of cell. One experiment involved mixing mitochondria with the same human cells that had donated the mitochondria. Another was mixing human mitochondria with rat muscle cells.
An important finding was that human mitochondria ended up in the rat muscle cells, but rat mitochondria did not increase in the muscle cells. This meant that mitochondria from outside the muscle cells was being successful delivered inside the cells.
When the team compared the efficiency of its mitochondria delivery method with a method called passive transfer, they discovered that their method was much more effective.
“The results from centrifugal transfer indicate that mitochondria can pass through the cell membrane more easily than using the passive transfer approach,” they wrote.
The team maintained the higher efficiency level when they used three other types of cells, including stem cells and cancer cells.
The key finding was that the transferred mitochondria were able to restore lost mitochondrial function, including generating energy and the reactive oxygen species that take part in metabolism.
“This simple and rapid mitochondrial transfer method can be used to treat mitochondrial dysfunction-related diseases,” the team concluded.
News source: https://mitochondrialdiseasenews.com
Photo credit: mitochondrialdiseasenews