Do mitochondria care about insulin resistance?
- Details
- Published on 21 October 2014

Dr. Chrysi Koliaki will present her last studies published in Molecular Metabolism.
In the last issue of Molecular Metabolism, Dr. Chrysi Koliaki addressed the intensively debated term “mitochondrial dysfunction” in the context of insulin resistance using a comprehensive cell-autonomous approach, which allowed constant substrate delivery to distinct insulin-sensitive cell types circumventing confounding effects of in vivo inter-tissue crosstalk.
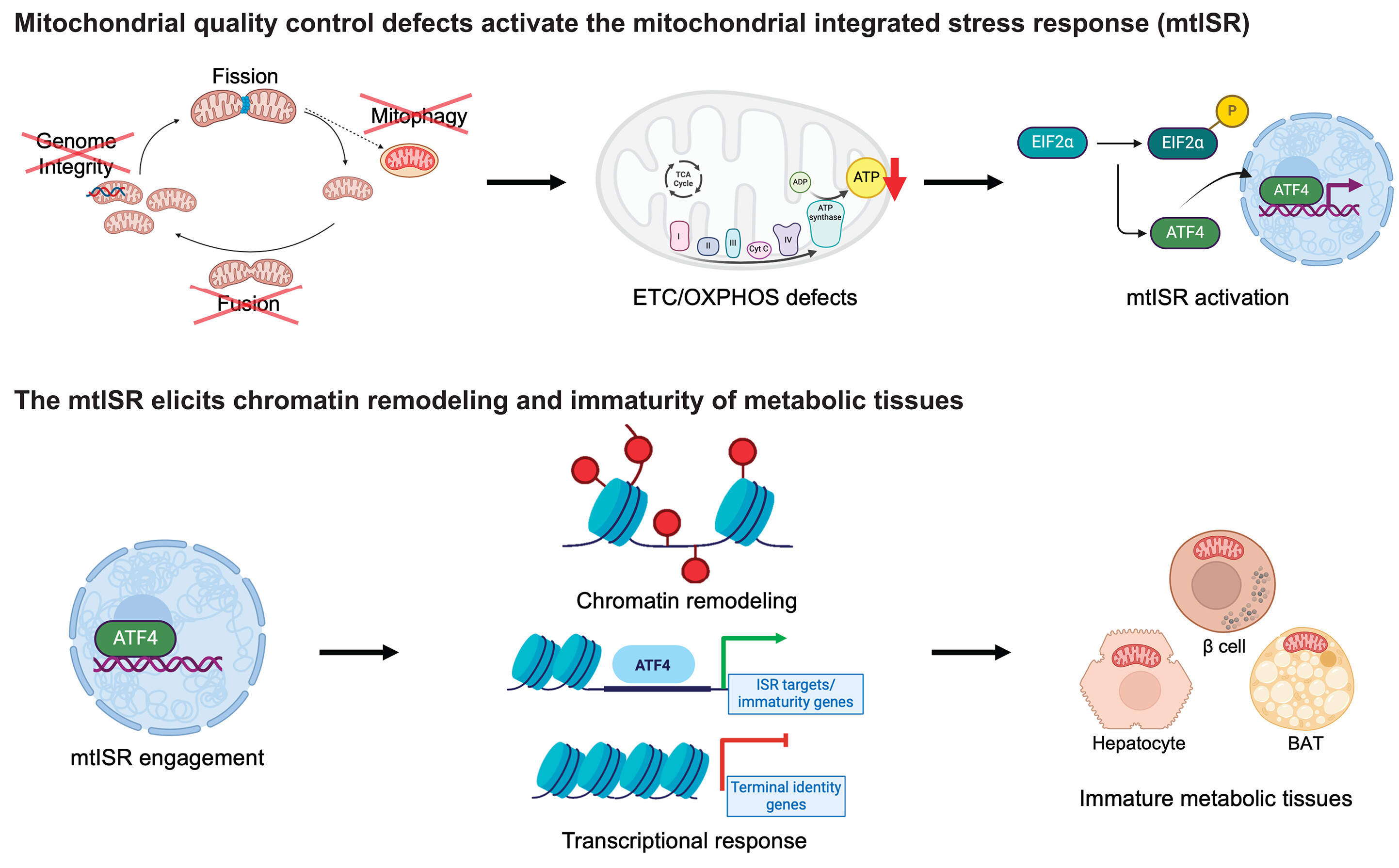
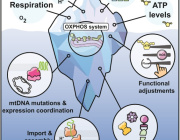
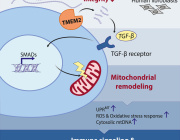
The authors provoked and titrated the reduction of oxidative phosphorylation (OXPHOS) by inhibiting respiratory complexes I and V in 3T3L1 adipocyte and FAO hepatoma cell cultures, and assessed the effects on insulin sensitivity. Inhibition of OXPHOS reduced the insulin action in adipocytes but had insulin-sensitizing effects in hepatocytes. Interestingly, these effects occurred independently of the production of reactive oxygen species (ROS).
Although using the misleading term “mitochondrial dysfunction”, which should be avoided as it implies one unifying abnormality of mitochondria, the authors took effort to assess several interdependent aspects of mitochondrial function such as respiration, membrane potential, and ROS production. An even more comprehensive assessment of mitochondrial function could have been obtained by integrating additional features into this multi-component analysis, such as β-oxidation, tricarboxylic acid (TCA) cycle activity, and mitochondrial content or density. Of note, it is of interest to examine whether their findings would also hold true for other cell types, such as the β-cell or the skeletal muscle cell, which is mainly responsible for insulin-mediated whole-body glucose disposal and oxidative adaptation to physical training as well as a key player in the pathogenesis of insulin resistance and type 2 diabetes mellitus (T2DM).
The finding of tissue-specific functional characteristics of mitochondria is not unexpected, as mitochondrial metabolism is tailored to meet the metabolic and bioenergetic demands of divergent tissues such as brain, heart, liver, adipose tissue, or skeletal muscle by means of their broad range of OXPHOS capacity. This diversity results from different combinations of mitochondrial content, amount of electron transport chain complexes, and their intrinsic activities, which can be viewed as an adaptation to the variable substrate fluxes and may further account for tissue-specific interference of mitochondrial function with insulin sensitivity.
The relationship between mitochondrial function and insulin sensitivity is influenced by various factors: (i) the tissue or cell type, as pointed by the study by Martin the mitochondrial features examined, (iii) the mode of insulin resistance, and (iv) time-dependent interaction.
First, the postulate that reduced mitochondrial function uniformly associates with insulin resistance is based on studies in human skeletal muscle in vivo and in vitro. Nevertheless, upregulated OXPHOS gene expression has been already found in liver of T2DM patients.
Second, inhibition of individual mitochondrial features such as β-oxidation vs. OXPHOS might cause opposing effects on insulin sensitivity. For example, mice exhibiting genetic defects related to impaired β-oxidation exhibit reduced hepatic insulin sensitivity, whereas mice with inherited OXPHOS defects can present with improved insulin sensitivity. It is also important to differentiate between the distinct components of one mitochondrial feature such as the respiratory complexes I–V. Martin et al. contribute to unraveling this diversity, by showing differential effects on insulin sensitivity in hepatocytes and adipocytes depending on selective inhibition of complex I or V. These findings might pave the way to novel individualized therapeutic approaches for improving insulin sensitivity, by targeting not only specific tissues and mitochondrial features, but also distinct mitochondrial proteins and their bioactive subunits.
Dr. Chrysi Koliaki will present his strategic studies during targeting Mitochondria 2014
www.targeting-mitochondria.com