A Novel PTD-mediated IVT-mRNA delivery platform developed for Protein Replacement Therapy (PRT) for genetic/metabolic disorders: the case of human fatal infantile cardioencephalomyopathy attributed to SCO2 mutations

Lefkothea C. Papadopoulou, Professor of Pharmacology, from Aristotle University of Thessaloniki, Greece, will join us this year to present her most recent findings on "A Novel PTD-mediated IVT-mRNA delivery platform developed for Protein Replacement Therapy (PRT) for genetic/metabolic disorders: the case of human fatal infantile cardioencephalomyopathy attributed to SCO2 mutations".
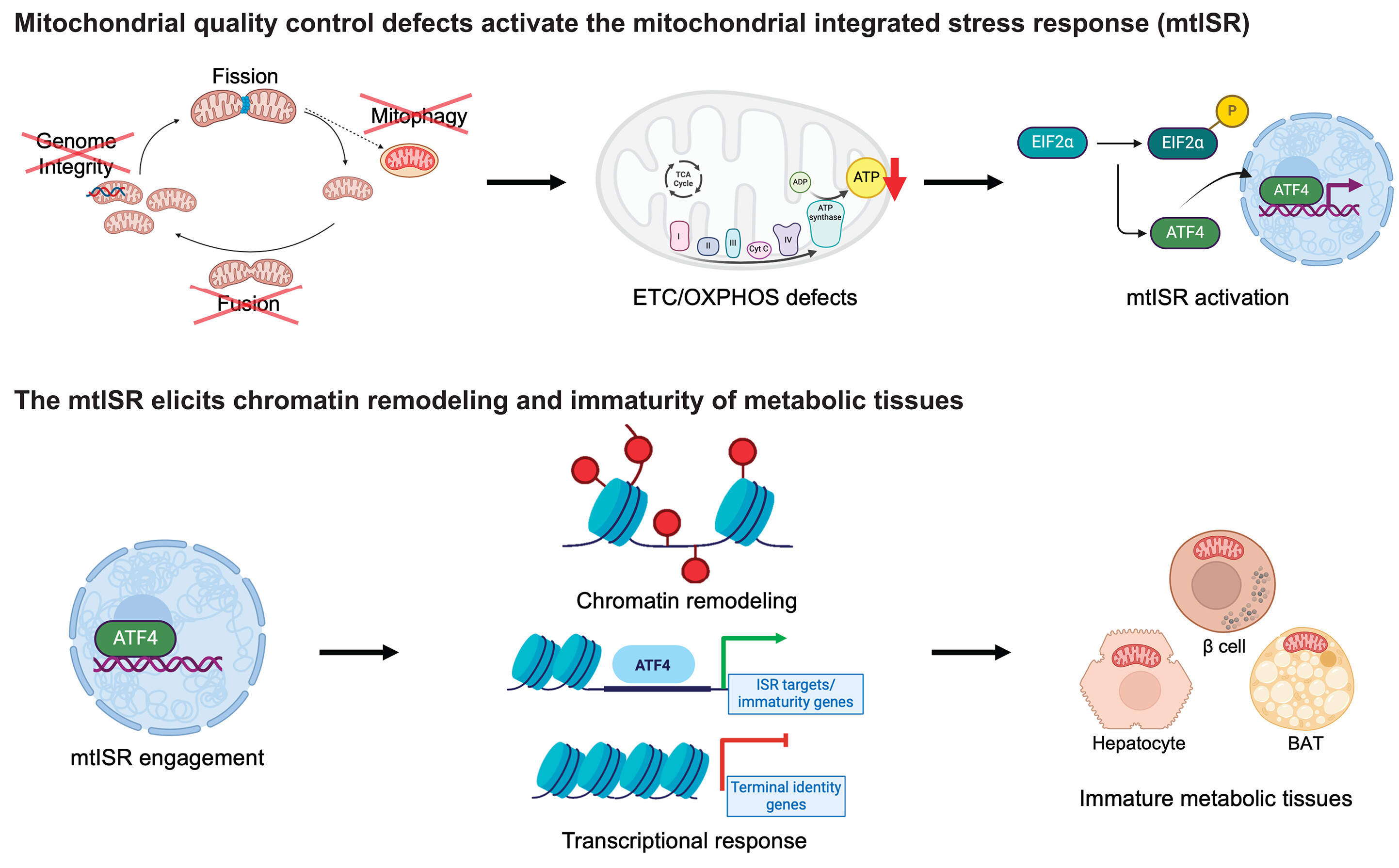
Mutations in human SCO2 gene, encoding the mitochondrial inner membrane Sco2 COX assembly protein, have been implicated in the mitochondrial disorder fatal infantile cardioencephalomyopathy accompanied by cytochrome c oxidase (COX) deficiency as well in neurodegenerative disorders, like Leigh syndrome.
Since there is no effective treatment for such disorders yet, a Protein Replacement Therapeutic (PRT) approach via the Protein Transduction domain (PTD) Technology was explored. Protein Transduction Domains (PTDs) or Cell Penetrating Peptides (CPPs), such as TAT, are small peptides, able to facilitate intracellular delivery of a variety of cargos, ranging from small molecules to macromolecules, and permit their localization in subcellular organelles, like mitochondria. In the frame of this work, Dr. Papadopoulou's group cloned the coding sequences of human SCO2 gene and expressed it in bacteria to produce the recombinant human TAT-L-Sco2 fusion protein.
Purified bacterial inclusion bodies, enriched in the fusion protein, were solubilized in 1M L-Arg solution. Exogenously administered TAT-L-Sco2 fusion protein was transduced successfully inside the mitochondria, where it was processed by MPP to yield the mature Sco2 protein, thus contributing to COX assembly and recovery of COX activity in fibroblasts derived from a SCO2/COX deficient patient. Radiolabeled TAT-L-Sco2 fusion protein, prepared and administered in animals (mice), was found to be biodistributed in peripheral tissues and successfully transduced into mitochondria in vivo.
Since the production of recombinant protein therapeutics requires complex purification procedures, increasing the cost of good manufacturing practice (GMP)’s protein products, Dr. Papadopoulou's group moved on to an alternative PRT approach, the PTD-mediated transduction of the vitro transcribed (IVT)-mRNAs. IVT-mRNA, produced in a cell-free system, resembles natural mRNA and its successful intracellular delivery permits transient expression / translation into the protein of interest.
The IVT-mRNA Technology platform has been optimized, in terms of IVT-mRNA structural stability, sensitivity to enzymatic degradation, inadequate translation and immunogenicity. Several structural and chemical modifications in IVT-mRNA increased the amount of protein produced per molecule of delivered mRNA, while reduced its immunogenicity. Furthermore, the potential clinical applications of the IVT-mRNAs strongly depend on their successful delivery to produce enough of the desired protein intracellularly. A wide range of in vitro and in vivo transfection reagents have been shown to facilitate IVT-mRNAs intracellular uptake, including protection from degradation.
Having all these in mind, Dr. Papadopoulou's group developed a novel PTD-mediated IVT-mRNA delivery platform [International Patent Pending, PCT/GR2020/000059; Greek patent: 1010063 (National)] as a protein therapy approach for metabolic / genetic disorders, using the amphipathic PTD, PFVYLI. The selected PTD is exploited as a carrier for any therapeutic IVT-mRNA through a novel, covalent reaction, with puromycin (conjugated via an amide bond to the PTD) served as a linker to the IVT-mRNA. PTD was successfully conjugated to IVT-mRNA of SCO2, as confirmed by NMR data analysis and a band shift assay compared to the corresponding naked IVT-mRNA.
The proposed PTD-IVT-mRNA delivery platform showed significant stability of the studied IVT-mRNA in various conditions, indicating that the PTD protects the IVT-mRNA from immediate nuclease digestion. PTD-IVT-mRNA of SCO2 was successfully transduced (via clathrin-mediated endocytosis) into fibroblasts derived from a SCO2/COX deficient patient and led to the recovery of reduced COX activity, compared to the naked IVT-mRNA as well the IVT-mRNA conjugated to a non-PTD, Control peptide.
These findings suggest that this novel PTD-IVT-mRNA delivery strategy could be a promising platform for effective gene expression/translation into a protein of interest, with the potential to be used in clinical trials as a PRT for metabolic/genetic disorders.
Targeting Mitochondria 2022 Congress
October 26-28, 2022 - Berlin, Germany
wms-site.com