Antioxidant anti-diabetic agents and tumor metastasis: An alert by Marvin Edeas
- Details
- Published on 07 June 2016
 Few months ago, Dr. Hui Wang from the Department of Endocrinology at Xinqiao Hospital, Third Military Medical University, Chongqing, China, published a strategic study in the journal of Science Translational Medicine, 2016 entitled "NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis".
Few months ago, Dr. Hui Wang from the Department of Endocrinology at Xinqiao Hospital, Third Military Medical University, Chongqing, China, published a strategic study in the journal of Science Translational Medicine, 2016 entitled "NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis".
According to Marvin Edeas from Institut Cochin, INSERM U1016, University Paris Descartes, we need to highlight the subtility and complexity of antioxidants in health and diseases. Marvin Edeas thinks that this study is one of the most hot study published in 2016 and that the impact of Dr Hui Wang's studies on cancer acceleration is and will stay very important.
In 2009, M. Edeas already commented about the negative effects of antioxidants supplementation: "We don't need to be supplemented by antioxidants each day." From his side, as scientific advisor of WMS and ISANH, Martin Bergö reviewed Dr Wang's paper and clearly demonstrated that antioxidants can accelerate many kinds of cancer.
The impact of antioxidant supplementation on malignant melanoma progression
On the role of ROS and antioxidants in tumor initiation and progression
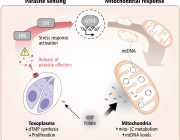
Anna Azvolinsky from the-scientist commented: Two classes of drugs used to treat type 2 diabetes can increase metastasis of human tumors implanted in mice, according to a study published today (April 13) in Science Translational Medicine. The ability of these two classes of compounds—hypoglycemic dipeptidyl peptidase–4 (DPP-4) inhibitors and antineuropathic α-lipoic acid (ALA)—to accelerate metastasis was due to activation of an antioxidant response pathway that prompted expression of metastasis-promoting proteins, scientists in China have shown. The team’s results add to a growing body of literature highlighting links between antioxidants and the spread of cancer.
“The most important finding is that some antidiabetic agents . . . promote the metastasis of existing cancers by activating [an antioxidant response],” wrote study coauthor Hongting Zheng of the Third Military Medical University in Chongqing, China.
“The data are compelling and the study is consistent with other studies that would argue antioxidant activity can be helpful for cancer cells to metastasize,” said cell biologist Zachary Schafer of the University of Notre Dame who was not involved in the work.
“What most surprised me are the similar effects on so many tumor cell lines [the authors studied],” said Martin Bergö, who studies the effects of antioxidants on the spread of melanoma and lung cancer at the Karolinska Institute in Sweden, but was not involved in the work. He will also present his excellent studies during Paris Redox 2016. www.isanh.net
Zheng and colleagues first screened commonly used antidiabetic drugs—including metformin and insulin analogs—for their abilities to boost proliferation or increase migration of cancer cells in vitro. The researchers showed that DPP-4 inhibitors promoted migration and invasion but not proliferation of melanoma, hepatic, colon, breast, lung, and ovarian human cancer cell lines.
In mice, these drugs resulted in greater dissemination of transplanted human liver and colon tumor cells as well as increased micrometastases compared to animals with the same tumors that received no antidiabetic drugs. Additional in vitro experiments suggested that the DPP-4 inhibitors’ effects on tumor cell migration were associated with the compounds’ abilities to reduce oxidative stress of cancer cells: the drugs reduced reactive oxygen species (ROS) and increased glutathione, an endogenous antioxidant. Adding a glutathione synthesis inhibitor to the cancer cells in addition to a DPP-4 inhibitor prevented the tumor cells from migrating, the researchers shows.
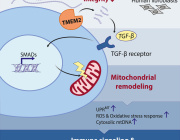
To understand how these compounds act on cellular oxidative stress pathways, the researchers zeroed in on the transcription factor nuclear factor E2–related factor 2 (NRF2), which they found to be activated by DPP-4 inhibitor treatment, both in cell culture and in mice. Five different DPP-4 inhibitors all activated NRF2 in vitro. When the researchers knocked down NRF2 in DPP-4 inhibitor–treated colon and a liver cancer cell lines, they saw a reduction in both tumor cell migration and in the expression of metastasis-associated proteins.
 The researchers saw a similar effect in vivo. Mice inoculated with NRF2 knockdown cells had fewer DPP-4 inhibitor–induced metastases. NRF2 also influenced metastasis independent of any diabetes drug treatment, the researchers reported. Activation of the transcription factor promoted the expression of metastasis proteins and cell migration in culture, and pharmacological activation of NRF2 in mice increased micrometastases.
The researchers saw a similar effect in vivo. Mice inoculated with NRF2 knockdown cells had fewer DPP-4 inhibitor–induced metastases. NRF2 also influenced metastasis independent of any diabetes drug treatment, the researchers reported. Activation of the transcription factor promoted the expression of metastasis proteins and cell migration in culture, and pharmacological activation of NRF2 in mice increased micrometastases.
Another known NRF2 activator, ALA—used to treat diabetic neuropathy—had similar effects as the DPP-4 inhibitors studied, both in vitro and in vivo.
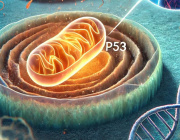
Analyzing primary and metastatic tumor expression data, the team found that metastatic samples were more likely to have increased NRF2 expression, and that NRF2 expression correlated with lymph node metastasis.
Previous studies have shown that NRF2 can be activated by oncogenes, allowing tumors to quench ROS that can impede their growth. Yet whether other drugs with antioxidant activity work by a similar mechanism, and whether cancer cells themselves also use other mechanisms to maintain an antioxidant state, are open questions, Schafer noted.
The next step is to study tumor metastasis in diabetic mice, which “will more adequately reflect the current clinical application of antidiabetic drugs,” Zheng wrote in an email.
“Antioxidants and drugs that activate NRF2 do not cause cancer,” Bergo stressed. “Rather, they help healthy cells stay healthy and they help cancer cells stay healthy and allow them to spread in the body.”
H. Wang et al., “NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis,” Science Translational Medicine, doi:10.1126/scitranslmed.aad6095, 2016.